Release and activity of histone in diseases
- PMID: 25118930
- PMCID: PMC4454312
- DOI: 10.1038/cddis.2014.337
Release and activity of histone in diseases
Abstract
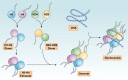
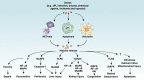
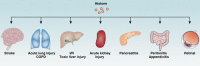
Histones and their post-translational modifications have key roles in chromatin remodeling and gene transcription. Besides intranuclear functions, histones act as damage-associated molecular pattern molecules when they are released into the extracellular space. Administration of exogenous histones to animals leads to systemic inflammatory and toxic responses through activating Toll-like receptors and inflammasome pathways. Anti-histone treatment (e.g., neutralizing antibodies, activated protein C, recombinant thrombomodulin, and heparin) protect mice against lethal endotoxemia, sepsis, ischemia/reperfusion injury, trauma, pancreatitis, peritonitis, stroke, coagulation, and thrombosis. In addition, elevated serum histone and nucleosome levels have been implicated in multiple pathophysiological processes and progression of diseases including autoimmune diseases, inflammatory diseases, and cancer. Therefore, extracellular histones could serve as biomarkers and novel therapeutic targets in human diseases.
Figures


References
-
- Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599. - PubMed
-
- Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–1016. - PubMed
-
- Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat Struct Mol Biol. 2013;20:259–266. - PubMed
-
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. - PubMed
-
- Allam R, Kumar SV, Darisipudi MN, Anders HJ. Extracellular histones in tissue injury and inflammation. J Mol Med (Berl) 2014;92:465–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

