Successful non-surgical deep uterine transfer of porcine morulae after 24 hour culture in a chemically defined medium
- PMID: 25118944
- PMCID: PMC4131926
- DOI: 10.1371/journal.pone.0104696
Successful non-surgical deep uterine transfer of porcine morulae after 24 hour culture in a chemically defined medium
Abstract
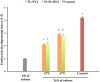
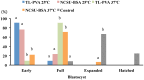
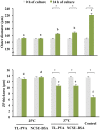
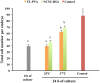
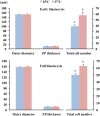
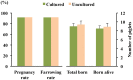
Excellent fertility and prolificacy have been reported after non-surgical deep uterine transfers of fresh in vivo-derived porcine embryos. Unfortunately, when this technology is used with vitrified embryos, the reproductive performance of recipients is low. For this reason and because the embryos must be stored until they are transferred to the recipient farms, we evaluated the potential application of non-surgical deep uterine transfers with in vivo-derived morulae cultured for 24 h in liquid stage. In Experiment 1, two temperatures (25 °C and 37 °C) and two media (one fully defined and one semi-defined) were assessed. Morulae cultured in culture medium supplemented with bovine serum albumin and fetal calf serum at 38.5 °C in 5% CO2 in air were used as controls. Irrespective of medium, the embryo viability after 24 h of culture was negatively affected (P<0.05) at 25 °C but not at 37 °C compared with the controls. Embryo development was delayed in all experimental groups compared with the control group (P<0.001). Most of the embryos (95.7%) cultured at 37 °C achieved the full or expanded blastocyst stage, and unlike the controls, none of them hatched at the end of culture. In Experiment 2, 785 morulae were cultured in the defined medium at 37 °C for 24 h, and the resulting blastocysts were transferred to the recipients (n = 24). Uncultured embryos collected at the blastocyst stage (n = 750) were directly transferred to the recipients and used as controls (n = 25). No differences in farrowing rates (91.7% and 92.0%) or litter sizes (9.0 ± 0.6 and 9.4 ± 0.8) were observed between the groups. This study demonstrated, for the first time, that high reproductive performance can be achieved after non-surgical deep uterine transfers with short-term cultured morulae in a defined medium, which opens new possibilities for the sanitary, safe national and international trade of porcine embryos and the commercial use of embryo transfer in pigs.
Conflict of interest statement
Figures
References
-
- Day BN (2000) Reproductive biotechnologies: current status in porcine reproduction. Anim Reprod Sci 60–61: 161–72. - PubMed
-
- Hazeleger W, Kemp B (2001) Recent developments in pig embryo transfer. Theriogenology 56(8): 1321–1331. - PubMed
-
- Martinez EA, Vazquez JM, Roca J, Lucas X, Gil MA, et al.. (2001) Deep intrauterine insemination and embryo transfer in pigs. Reproduction 58 (suppl): 301–311. - PubMed
-
- Cameron RDA, Durack M, Fogarty R, Putra DKH, McVeigh J (1989) Practical experience with commercial embryo transfer in pigs. Aust Vet J 66(10): 314–318. - PubMed
-
- Martinez EA, Caamaño JN, Gil MA, Rieke A, Mccauley TC, et al. (2004) Successful nonsurgical deep uterine embryo transfer in pigs. Theriogenology 61(1): 137–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

