Enhancing Patient Flexibility of Subcutaneous Immunoglobulin G Dosing: Pharmacokinetic Outcomes of Various Maintenance and Loading Regimens in the Treatment of Primary Immunodeficiency
- PMID: 25118975
- PMCID: PMC4254869
- DOI: 10.1007/s13554-014-0018-0
Enhancing Patient Flexibility of Subcutaneous Immunoglobulin G Dosing: Pharmacokinetic Outcomes of Various Maintenance and Loading Regimens in the Treatment of Primary Immunodeficiency
Abstract
Introduction: Standard treatment for patients with primary immunodeficiency (PID) is monthly intravenous immunoglobulin (IVIG), or weekly/biweekly subcutaneous immunoglobulin (SCIG) infusion. We used population pharmacokinetic modeling to predict immunoglobulin G (IgG) exposure following a broad range of SCIG dosing regimens for initiation and maintenance therapy in patients with PID.
Methods: Simulations of SCIG dosing were performed to predict IgG concentration-time profiles and exposure metrics [steady-state area under the IgG concentration-time curve (AUC), IgG peak concentration (C max), and IgG trough concentration (C min) ratios] for various infusion regimens.
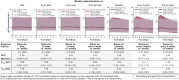
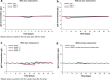
Results: The equivalent of a weekly SCIG maintenance dose administered one, two, three, five, or seven times per week, or biweekly produced overlapping steady-state concentration-time profiles and similar AUC, C max, and C min values [95% confidence interval (CI) for ratios was 0.98-1.03, 0.95-1.09, and 0.92-1.08, respectively]. Administration every 3 or 4 weeks resulted in higher peaks and lower troughs; the 95% CI of the AUC, C max, and C min ratios was 0.97-1.04, 1.07-1.26, and 0.86-0.95, respectively. IgG levels >7 g/L were reached within 1 week using a loading dose regimen in which the weekly maintenance dose was administered five times in the first week of treatment. In patients with very low endogenous IgG levels, administering 1.5 times the weekly maintenance dose five times in the first week of treatment resulted in a similar response.
Conclusions: The same total weekly SCIG dose can be administered at different intervals, from daily to biweekly, with minimal impact on serum IgG levels. Several SCIG loading regimens rapidly achieve adequate serum IgG levels in treatment-naïve patients.
Keywords: Biological therapy; Dosing regimen; Hizentra®; Immunoglobulin replacement therapy; Loading dose; Pharmacokinetic model; Primary immunodeficiency; Skipped doses; Subcutaneous immunoglobulin.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

