The gut microbiota and developmental programming of the testis in mice
- PMID: 25118984
- PMCID: PMC4132106
- DOI: 10.1371/journal.pone.0103809
The gut microbiota and developmental programming of the testis in mice
Abstract
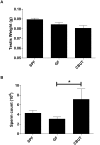
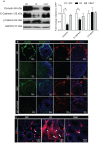
Nutrients and environmental chemicals, including endocrine disruptors, have been incriminated in the current increase in male reproductive dysfunction, but the underlying mechanisms remain unknown. The gastrointestinal tract represents the largest surface area exposed to our environment and thereby plays a key role in connection with exposure of internal organs to exogenous factors. In this context the gut microbiome (all bacteria and their metabolites) have been shown to be important contributors to body physiology including metabolism, cognitive functions and immunity. Pivotal to male reproduction is a proper development of the testis, including the formation of the blood-testis barrier (BTB) that encapsulates and protects germ cells from stress induced environmental cues, e.g. pathogenic organisms and xenobiotics. Here we used specific pathogen free (SPF) mice and germ-free (GF) mice to explore whether gut microbiota and/or their metabolites can influence testis development and regulation of BTB. Lumen formation in the seminiferous tubules, which coincides with the development of the BTB was delayed in the testes of GF mice at 16 days postpartum. In addition, perfusion experiments (Evans blue) demonstrated increased BTB permeability in these same mice. Reduced expressions of occludin, ZO-2 and E-cadherin in GF testis suggested that the microbiota modulated BTB permeability by regulation of cell-cell adhesion. Interestingly, exposure of GF mice to Clostridium Tyrobutyricum (CBUT), which secrete high levels of butyrate, restored the integrity of the BTB and normalized the levels of cell adhesion proteins. Moreover, the GF mice exhibited lower serum levels of gonadotropins (LH and FSH) than the SPF group. In addition, the intratesticular content of testosterone was lower in GF compared to SPF or CBUT animals. Thus, the gut microbiome can modulate the permeability of the BTB and might play a role in the regulation of endocrine functions of the testis.
Conflict of interest statement
Figures



References
-
- Petersen C, Soder O (2006) The sertoli cell–a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res 66: 153–161. - PubMed
-
- Hess RA, Renato de Franca L (2008) Spermatogenesis and cycle of the seminiferous epithelium. Advances in experimental medicine and biology 636: 1–15. - PubMed
-
- Cheng CY, Mruk DD (2002) Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiological reviews 82: 825–874. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

