Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function
- PMID: 25119024
- PMCID: PMC4376130
- DOI: 10.1038/nature13611
Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function
Abstract
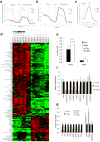
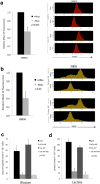
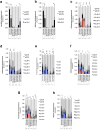
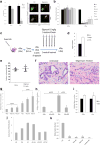
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest cancers in western countries, with a median survival of 6 months and an extremely low percentage of long-term surviving patients. KRAS mutations are known to be a driver event of PDAC, but targeting mutant KRAS has proved challenging. Targeting oncogene-driven signalling pathways is a clinically validated approach for several devastating diseases. Still, despite marked tumour shrinkage, the frequency of relapse indicates that a fraction of tumour cells survives shut down of oncogenic signalling. Here we explore the role of mutant KRAS in PDAC maintenance using a recently developed inducible mouse model of mutated Kras (Kras(G12D), herein KRas) in a p53(LoxP/WT) background. We demonstrate that a subpopulation of dormant tumour cells surviving oncogene ablation (surviving cells) and responsible for tumour relapse has features of cancer stem cells and relies on oxidative phosphorylation for survival. Transcriptomic and metabolic analyses of surviving cells reveal prominent expression of genes governing mitochondrial function, autophagy and lysosome activity, as well as a strong reliance on mitochondrial respiration and a decreased dependence on glycolysis for cellular energetics. Accordingly, surviving cells show high sensitivity to oxidative phosphorylation inhibitors, which can inhibit tumour recurrence. Our integrated analyses illuminate a therapeutic strategy of combined targeting of the KRAS pathway and mitochondrial respiration to manage pancreatic cancer.
Figures
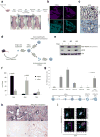
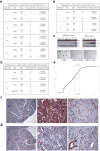
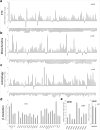
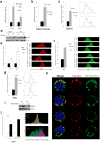
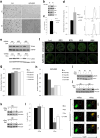
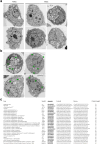
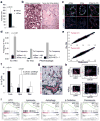
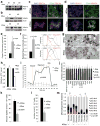


Comment in
-
Targeted therapy: Targeting mitochondria in pancreatic cancer.Nat Rev Clin Oncol. 2014 Oct;11(10):562. doi: 10.1038/nrclinonc.2014.143. Epub 2014 Aug 26. Nat Rev Clin Oncol. 2014. PMID: 25155173 No abstract available.
References
-
- NCI/SEER. 2012 http://seer.cancer.gov/statfacts/html/pancreas.html - incidence-mortality.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

