Onset of analgesia and efficacy of ibuprofen sodium in postsurgical dental pain: a randomized, placebo-controlled study versus standard ibuprofen
- PMID: 25119511
- PMCID: PMC4388398
- DOI: 10.1097/AJP.0000000000000142
Onset of analgesia and efficacy of ibuprofen sodium in postsurgical dental pain: a randomized, placebo-controlled study versus standard ibuprofen
Abstract
Objectives: A novel, immediate-release tablet formulation of ibuprofen (IBU) sodium dihydrate, Advil Film Coated Tablets (IBUNa), has been developed that is absorbed faster than standard IBU tablets. The objective of the current study was to compare the efficacy and onset of analgesia of this new formulation with standard IBU tablets after a single dose.
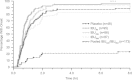
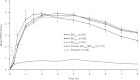
Materials and methods: Patients (N=316) with at least moderate baseline postsurgical dental pain were randomized to 400 mg IBUNa, Advil (IBUAdv), Motrin (IBUMot), or placebo. Primary endpoints were time-weighted sum of pain relief (PR) and pain intensity differences over 8 hours (SPRID 0-8) and time to onset of meaningful pain relief (TMPR) measured by the double-stopwatch method.
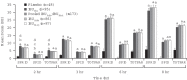
Results: SPRID 0-8 was significantly greater for IBUNa and the other active treatments versus placebo (P<0.001). IBUNa had a significantly earlier TMPR versus placebo, pooled IBUAdv/IBUMot, and IBUMot (P<0.001 for all), and a marginally faster TMPR (P=0.075) versus IBUAdv. Results for secondary endpoints were similar. Adverse events were comparable across treatment groups, with gastrointestinal disorders being most frequently reported. Most adverse events were mild or moderate.
Discussion: This novel formulation of IBUNa provided superior overall PR compared with placebo and more rapid onset of analgesic effect compared with standard IBU tablets. Rapid PR is important in the treatment of acute pain, including dental pain, and this IBUNa formulation represents a new treatment option for rapid PR.
Trial registration: ClinicalTrials.gov NCT01098747.
Conflict of interest statement
This study was sponsored by Pfizer Madison, NJ, USA. Financial support for medical editorial assistance was provided by Pfizer. R.L. and D.K. are employees of Pfizer Consumer Healthcare. G.D. is a former employee of Pfizer Consumer Healthcare. P.B. has received grant/research support from Jean Brown Research. P.B. declares no conflict of interest.
Figures

References
-
- Advil [package insert]. Madison: Pfizer Consumer Healthcare, 2012.
-
- Motrin IB. [package insert]. Fort Washington, PA: McNeil Consumer Healthcare, a division of McNEIL-PPC Inc.; 2013.
-
- Braun RP, Lockhart EA, Bruno P. Delayed-onset muscle soreness (DOMS)—a new pain model to compare OTC analgesics [abstract 79]. Med Sci Sports Exerc. 1994;26suppl 5S14.
-
- Cooper SA, Schachtel BP, Goldman E, et al. Ibuprofen and acetaminophen in the relief of acute pain: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 1989;29:1026–1030. - PubMed
-
- Schachtel BP, Fillingim JM, Thoden WR, et al. Sore throat pain in the evaluation of mild analgesics. Clin Pharmacol Ther. 1988;44:704–711. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

