Telomerase inhibition abolishes the tumorigenicity of pediatric ependymoma tumor-initiating cells
- PMID: 25120190
- PMCID: PMC4286630
- DOI: 10.1007/s00401-014-1327-6
Telomerase inhibition abolishes the tumorigenicity of pediatric ependymoma tumor-initiating cells
Abstract
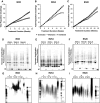
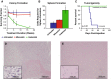
Pediatric ependymomas are highly recurrent tumors resistant to conventional chemotherapy. Telomerase, a ribonucleoprotein critical in permitting limitless replication, has been found to be critically important for the maintenance of tumor-initiating cells (TICs). These TICs are chemoresistant, repopulate the tumor from which they are identified, and are drivers of recurrence in numerous cancers. In this study, telomerase enzymatic activity was directly measured and inhibited to assess the therapeutic potential of targeting telomerase. Telomerase repeat amplification protocol (TRAP) (n = 36) and C-circle assay/telomere FISH/ATRX staining (n = 76) were performed on primary ependymomas to determine the prevalence and prognostic potential of telomerase activity or alternative lengthening of telomeres (ALT) as telomere maintenance mechanisms, respectively. Imetelstat, a phase 2 telomerase inhibitor, was used to elucidate the effect of telomerase inhibition on proliferation and tumorigenicity in established cell lines (BXD-1425EPN, R254), a primary TIC line (E520) and xenograft models of pediatric ependymoma. Over 60 % of pediatric ependymomas were found to rely on telomerase activity to maintain telomeres, while no ependymomas showed evidence of ALT. Children with telomerase-active tumors had reduced 5-year progression-free survival (29 ± 11 vs 64 ± 18 %; p = 0.03) and overall survival (58 ± 12 vs 83 ± 15 %; p = 0.05) rates compared to those with tumors lacking telomerase activity. Imetelstat inhibited proliferation and self-renewal by shortening telomeres and inducing senescence in vitro. In vivo, Imetelstat significantly reduced subcutaneous xenograft growth by 40 % (p = 0.03) and completely abolished the tumorigenicity of pediatric ependymoma TICs in an orthotopic xenograft model. Telomerase inhibition represents a promising therapeutic approach for telomerase-active pediatric ependymomas found to characterize high-risk ependymomas.
Figures



References
-
- Abedalthagafi M, Phillips JJ, Kim GE, Mueller S, Haas-Kogen DA, Marshall RE, Croul SE, Santi MR, Cheng J, Zhou S, Sullivan LM, Martinez-Lage M, Judkins AR, Perry A. The alternative lengthening of telomere phenotype is significantly associated with loss of ATRX expression in high-grade pediatric and adult astrocytomas: a multi-institutional study of 214 astrocytomas. Mod Pathol. 2013;26(11):1425–1432. doi: 10.1038/modpathol.2013.90. - DOI - PMC - PubMed
-
- Castelo-Branco P, Choufani S, Mack S, Gallagher D, Zhang C, Lipman T, Zhukova N, Walker EJ, Martin D, Merino D, Wasserman JD, Elizabeth C, Alon N, Zhang L, Hovestadt V, Kool M, Jones DT, Zadeh G, Croul S, Hawkins C, Hitzler J, Wang JC, Baruchel S, Dirks PB, Malkin D, Pfister S, Taylor MD, Weksberg R, Tabori U. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14(6):534–542. doi: 10.1016/S1470-2045(13)70110-4. - DOI - PubMed
-
- Castelo-Branco P, Zhang C, Lipman T, Fujitani M, Hansford L, Clarke I, Harley CB, Tressler R, Malkin D, Walker E, Kaplan DR, Dirks P, Tabori U. Neural tumor-initiating cells have distinct telomere maintenance and can be safely targeted for telomerase inhibition. Clin Cancer Res. 2011;17(1):111–121. doi: 10.1158/1078-0432.CCR-10-2075. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

