Clinical and economic studies of eptifibatide in coronary stenting
- PMID: 25120366
- PMCID: PMC4128842
- DOI: 10.2147/TCRM.S35664
Clinical and economic studies of eptifibatide in coronary stenting
Erratum in
-
Erratum: Clinical and economic studies of eptifibatide in coronary stenting [Corrigendum].Ther Clin Risk Manag. 2014 Oct 28;10:913. doi: 10.2147/TCRM.S75813. eCollection 2014. Ther Clin Risk Manag. 2014. PMID: 25412931 Free PMC article.
Abstract
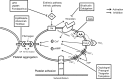
Platelet adhesion and aggregation at the site of coronary stenting can have catastrophic clinical and economic consequences. Therefore, effective platelet inhibition is vital during and after percutaneous coronary intervention. Eptifibatide is an intravenous antiplatelet agent that blocks the final common pathway of platelet aggregation and thrombus formation by binding to glycoprotein IIb/IIIa receptors on the surface of platelets. In clinical studies, eptifibatide was associated with a significant reduction of mortality, myocardial infarction, or target vessel revascularization in patients with acute coronary syndrome undergoing percutaneous coronary intervention. However, recent trials conducted in the era of dual antiplatelet therapy and newer anticoagulants failed to demonstrate similar results. The previously seen favorable benefit of eptifibatide was mainly offset by the increased risk of bleeding. Current American College of Cardiology/American Heart Association guidelines recommend its use as an adjunct in high-risk patients who are undergoing percutaneous coronary intervention with traditional anticoagulants (heparin or enoxaparin), who are not otherwise at high risk of bleeding. In patients receiving bivalirudin (a newer safer anticoagulant), routine use of eptifibatide is discouraged except in select situations (eg, angiographic complications). Although older pharmacoeconomic studies favor eptifibatide, in the current era of P2Y12 inhibitors and newer safer anticoagulants, the increased costs associated with bleeding make the routine use of eptifibatide an economically nonviable option. The cost-effectiveness of eptifibatide with the use of strategies that decrease the bleeding risk (eg, transradial access) is unknown. This review provides an overview of key clinical and economic studies of eptifibatide well into the current era of potent antiplatelet agents, novel safer anticoagulants, and contemporary percutaneous coronary intervention.
Keywords: Integrilin®; acute coronary syndrome; coronary artery disease; cost-effectiveness; eptifibatide; glycoprotein IIb/IIIa inhibitors; percutaneous coronary intervention.
Figures
References
-
- Nordmann AJ, Hengstler P, Leimenstoll BM, Harr T, Young J, Bucher HC. Clinical outcomes of stents versus balloon angioplasty in non-acute coronary artery disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2004;25(1):69–80. - PubMed
-
- van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53(16):1399–1409. - PubMed
-
- Gurbel PA, Cummings CC, Bell CR, Alford AB, Meister AF, Serebruany VL. Onset and extent of platelet inhibition by clopidogrel loading in patients undergoing elective coronary stenting: the Plavix Reduction Of New Thrombus Occurrence (PRONTO) trial. Am Heart J. 2003;145(2):239–247. - PubMed
-
- Steinhubl SR, Ellis SG, Wolski K, Lincoff AM, Topol EJ. Ticlopidine pretreatment before coronary stenting is associated with sustained decrease in adverse cardiac events: data from the Evaluation of Platelet IIb/IIIa Inhibitor for Stenting (EPISTENT) Trial. Circulation. 2001;103(10):1403–1409. - PubMed
-
- Cutlip DE, Baim DS, Ho KKL, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103(15):1967–1971. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

