Inhibitory projections from the ventral nucleus of the trapezoid body to the medial nucleus of the trapezoid body in the mouse
- PMID: 25120436
- PMCID: PMC4114201
- DOI: 10.3389/fncir.2014.00083
Inhibitory projections from the ventral nucleus of the trapezoid body to the medial nucleus of the trapezoid body in the mouse
Abstract
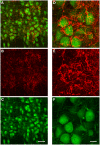
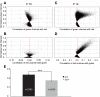
Neurons in the medial nucleus of the trapezoid body (MNTB) receive prominent excitatory input through the calyx of Held, a giant synapse that produces large and fast excitatory currents. MNTB neurons also receive inhibitory glycinergic inputs that are also large and fast, and match the calyceal excitation in terms of synaptic strength. GABAergic inputs provide additional inhibition to MNTB neurons. Inhibitory inputs to MNTB modify spiking of MNTB neurons both in-vitro and in-vivo, underscoring their importance. Surprisingly, the origin of the inhibitory inputs to MNTB has not been shown conclusively. We performed retrograde tracing, anterograde tracing, immunohistochemical experiments, and electrophysiological recordings to address this question. The results support the ventral nucleus of the trapezoid body (VNTB) as at least one major source of glycinergic input to MNTB. VNTB fibers enter the ipsilateral MNTB, travel along MNTB principal neurons and produce several bouton-like presynaptic terminals. Further, the contribution of GABA to the total inhibition declines during development, resulting in only a very minor fraction of GABAergic inhibition in adulthood, which is matched in time by a reduction in expression of a GABA synthetic enzyme in VNTB principal neurons.
Keywords: MNTB; VNTB; auditory; calyx of held; glycinergic; inhibition; trapezoid body.
Figures
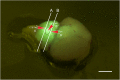
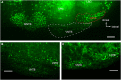
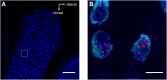

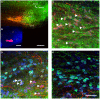




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

