Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research
- PMID: 25120485
- PMCID: PMC4112793
- DOI: 10.3389/fphar.2014.00174
Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research
Abstract
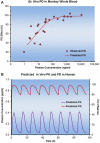
Characterizing the relationship between the pharmacokinetics (PK, concentration vs. time) and pharmacodynamics (PD, effect vs. time) is an important tool in the discovery and development of new drugs in the pharmaceutical industry. The purpose of this publication is to serve as a guide for drug discovery scientists toward optimal design and conduct of PK/PD studies in the research phase. This review is a result of the collaborative efforts of DMPK scientists from various Metabolism and Pharmacokinetic (MAP) departments of the global organization Novartis Institute of Biomedical Research (NIBR). We recommend that PK/PD strategies be implemented in early research phases of drug discovery projects to enable successful transition to drug development. Effective PK/PD study design, analysis, and interpretation can help scientists elucidate the relationship between PK and PD, understand the mechanism of drug action, and identify PK properties for further improvement and optimal compound design. Additionally, PK/PD modeling can help increase the translation of in vitro compound potency to the in vivo setting, reduce the number of in vivo animal studies, and improve translation of findings from preclinical species into the clinical setting. This review focuses on three important elements of successful PK/PD studies, namely partnership among key scientists involved in the study execution; parameters that influence study designs; and data analysis and interpretation. Specific examples and case studies are highlighted to help demonstrate key points for consideration. The intent is to provide a broad PK/PD foundation for colleagues in the pharmaceutical industry and serve as a tool to promote appropriate discussions on early research project teams with key scientists involved in PK/PD studies.
Keywords: DMPK; Novartis; PK-PD modeling; drug discovery; pharmacodynamics; pharmacokinetics.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

