Estrogens and stem cells in thyroid cancer
- PMID: 25120531
- PMCID: PMC4110518
- DOI: 10.3389/fendo.2014.00124
Estrogens and stem cells in thyroid cancer
Abstract
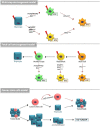
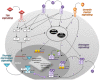
Recent discoveries highlight the emerging role of estrogens in the initiation and progression of different malignancies through their interaction with stem cell (SC) compartment. Estrogens play a relevant role especially for those tumors bearing a gender disparity in incidence and aggressiveness, as occurs for most thyroid diseases. Although several experimental lines suggest that estrogens promote thyroid cell proliferation and invasion, their precise contribution in SC compartment still remains unclear. This review underlines the interplay between hormones and thyroid function, which could help to complete the puzzle of gender discrepancy in thyroid malignancies. Defining the association between estrogen receptors' status and signaling pathways by which estrogens exert their effects on thyroid cells is a potential tool that provides important insights in pathogenetic mechanisms of thyroid tumors.
Keywords: cancer stem cells; estrogens; growth factors; stem cells; thyroid cancer; thyroid hormones.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

