Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage
- PMID: 25120903
- PMCID: PMC4130123
- DOI: 10.1186/2045-8118-11-18
Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage
Abstract
This article reviews current knowledge of the mechanisms underlying the initial hemorrhage and secondary blood-brain barrier (BBB) dysfunction in primary spontaneous intracerebral hemorrhage (ICH) in adults. Multiple etiologies are associated with ICH, for example, hypertension, Alzheimer's disease, vascular malformations and coagulopathies (genetic or drug-induced). After the initial bleed, there can be continued bleeding over the first 24 hours, so-called hematoma expansion, which is associated with adverse outcomes. A number of clinical trials are focused on trying to limit such expansion. Significant progress has been made on the causes of BBB dysfunction after ICH at the molecular and cell signaling level. Blood components (e.g. thrombin, hemoglobin, iron) and the inflammatory response to those components play a large role in ICH-induced BBB dysfunction. There are current clinical trials of minimally invasive hematoma removal and iron chelation which may limit such dysfunction. Understanding the mechanisms underlying the initial hemorrhage and secondary BBB dysfunction in ICH is vital for developing methods to prevent and treat this devastating form of stroke.
Keywords: Blood–brain barrier; Endothelium; Hematoma expansion; Hemoglobin; Intracerebral hemorrhage; Iron; Thrombin; Tight junction.
Figures
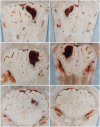
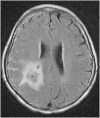



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

