The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation
- PMID: 25120911
- PMCID: PMC4130129
- DOI: 10.1038/bonekey.2014.56
The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation
Erratum in
-
Erratum: The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation.Bonekey Rep. 2016 Nov 30;5:856. doi: 10.1038/bonekey.2016.88. eCollection 2016. Bonekey Rep. 2016. PMID: 27974967 Free PMC article.
Abstract
The reversal phase couples bone resorption to bone formation by generating an osteogenic environment at remodeling sites. The coupling mechanism remains poorly understood, despite the identification of a number of 'coupling' osteogenic molecules. A possible reason is the poor attention for the cells leading to osteogenesis during the reversal phase. This review aims at creating awareness of these cells and their activities in adult cancellous bone. It relates cell events (i) on the bone surface, (ii) in the mesenchymal envelope surrounding the bone marrow and appearing as a canopy above remodeling surfaces and (iii) in the bone marrow itself within a 50-μm distance of this canopy. When bone remodeling is initiated, osteoprogenitors at these three different levels are activated, likely as a result of a rearrangement of cell-cell and cell-matrix interactions. Notably, canopies are brought under the osteogenic influence of capillaries and osteoclasts, whereas bone surface cells become exposed to the eroded matrix and other osteoclast products. In several diverse pathophysiological situations, including osteoporosis, a decreased availability of osteoprogenitors from these local reservoirs coincides with decreased osteoblast recruitment and impaired initiation of bone formation, that is, uncoupling. Overall, this review stresses that coupling does not only depend on molecules able to activate osteogenesis, but that it also demands the presence of osteoprogenitors and ordered cell rearrangements at the remodeling site. It points to protection of local osteoprogenitors as a critical strategy to prevent bone loss.
Conflict of interest statement
The author declares no conflict of interest.
Figures

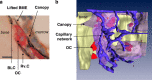

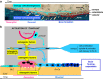
Similar articles
-
A joined role of canopy and reversal cells in bone remodeling--lessons from glucocorticoid-induced osteoporosis.Bone. 2015 Apr;73:16-23. doi: 10.1016/j.bone.2014.12.004. Epub 2014 Dec 10. Bone. 2015. PMID: 25497571
-
Re-thinking the bone remodeling cycle mechanism and the origin of bone loss.Bone. 2020 Dec;141:115628. doi: 10.1016/j.bone.2020.115628. Epub 2020 Sep 10. Bone. 2020. PMID: 32919109 Review.
-
A supra-cellular model for coupling of bone resorption to formation during remodeling: lessons from two bone resorption inhibitors affecting bone formation differently.Biochem Biophys Res Commun. 2014 Jan 10;443(2):694-9. doi: 10.1016/j.bbrc.2013.12.036. Epub 2013 Dec 12. Biochem Biophys Res Commun. 2014. PMID: 24333871
-
Osteoblast recruitment routes in human cancellous bone remodeling.Am J Pathol. 2014 Mar;184(3):778-89. doi: 10.1016/j.ajpath.2013.11.022. Epub 2014 Jan 9. Am J Pathol. 2014. PMID: 24412092
-
Osteoclasts Provide Coupling Signals to Osteoblast Lineage Cells Through Multiple Mechanisms.Annu Rev Physiol. 2020 Feb 10;82:507-529. doi: 10.1146/annurev-physiol-021119-034425. Epub 2019 Sep 25. Annu Rev Physiol. 2020. PMID: 31553686 Review.
Cited by
-
Age-related reference curves of volumetric bone density, structure, and biomechanical parameters adjusted for weight and height in a population of healthy women: an HR-pQCT study.Osteoporos Int. 2017 Apr;28(4):1335-1346. doi: 10.1007/s00198-016-3876-0. Epub 2016 Dec 15. Osteoporos Int. 2017. PMID: 27981337
-
Overcoming barriers confronting application of protein therapeutics in bone fracture healing.Drug Deliv Transl Res. 2021 Jun;11(3):842-865. doi: 10.1007/s13346-020-00829-x. Drug Deliv Transl Res. 2021. PMID: 32783153 Review.
-
Functional Roles of Netrin-1 in Osteoblast Differentiation.In Vivo. 2017 May-Jun;31(3):321-328. doi: 10.21873/invivo.11062. In Vivo. 2017. PMID: 28438858 Free PMC article.
-
CD146/MCAM defines functionality of human bone marrow stromal stem cell populations.Stem Cell Res Ther. 2016 Jan 11;7:4. doi: 10.1186/s13287-015-0266-z. Stem Cell Res Ther. 2016. PMID: 26753846 Free PMC article.
-
Disturbance of osteonal bone remodeling and high tensile stresses on the lateral cortex in atypical femoral fracture after long-term treatment with Risedronate and Alfacalcidol for osteoporosis.Bone Rep. 2021 May 7;14:101091. doi: 10.1016/j.bonr.2021.101091. eCollection 2021 Jun. Bone Rep. 2021. PMID: 34036125 Free PMC article.
References
-
- Frost HM. Dynamics of bone remodeling. In: Frost HM (ed) Bone Biodynamics Little, Brown and Co: Boston, MA, USA, 1964; p315–333.
-
- Dirckx N, Van HM, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today 2013;99:170–191. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
