Schistosome feeding and regurgitation
- PMID: 25121497
- PMCID: PMC4133383
- DOI: 10.1371/journal.ppat.1004246
Schistosome feeding and regurgitation
Abstract
Schistosomes are parasitic flatworms that infect >200 million people worldwide, causing the chronic, debilitating disease schistosomiasis. Unusual among parasitic helminths, the long-lived adult worms, continuously bathed in blood, take up nutrients directly across the body surface and also by ingestion of blood into the gut. Recent proteomic analyses of the body surface revealed the presence of hydrolytic enzymes, solute, and ion transporters, thus emphasising its metabolic credentials. Furthermore, definition of the molecular mechanisms for the uptake of selected metabolites (glucose, certain amino acids, and water) establishes it as a vital site of nutrient acquisition. Nevertheless, the amount of blood ingested into the gut per day is considerable: for males ∼100 nl; for the more actively feeding females ∼900 nl, >4 times body volume. Ingested erythrocytes are lysed as they pass through the specialized esophagus, while leucocytes become tethered and disabled there. Proteomics and transcriptomics have revealed, in addition to gut proteases, an amino acid transporter in gut tissue and other hydrolases, ion, and lipid transporters in the lumen, implicating the gut as the site for acquisition of essential lipids and inorganic ions. The surface is the principal entry route for glucose, whereas the gut dominates amino acid acquisition, especially in females. Heme, a potentially toxic hemoglobin degradation product, accumulates in the gut and, since schistosomes lack an anus, must be expelled by the poorly understood process of regurgitation. Here we place the new observations on the proteome of body surface and gut, and the entry of different nutrient classes into schistosomes, into the context of older studies on worm composition and metabolism. We suggest that the balance between surface and gut in nutrition is determined by the constraints of solute diffusion imposed by differences in male and female worm morphology. Our conclusions have major implications for worm survival under immunological or pharmacological pressure.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
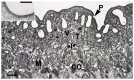
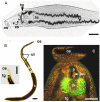
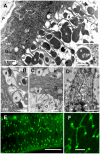

References
-
- Cheng TC, Bier JW (1972) Studies on molluscan schistosomiasis: an analysis of the development of the cercaria of Schistosoma mansoni. Parasitology 64: 129–141. - PubMed
-
- Hockley DJ (1972) Schistosoma mansoni: the development of the cercarial tegument. Parasitology 64: 245–252. - PubMed
-
- McLaren DJ, Hockley DJ (1977) Blood flukes have a double outer membrane. Nature 269: 147–149. - PubMed
-
- Fripp PJ (1967) The sites of (1-14C) glucose assimilation in Schistosoma haematobium. Comp Biochem Physiol 23: 893–898. - PubMed
-
- Skelly PJ, Tielens AGM, Shoemaker CB (1998) Glucose transport and metabolism in mammalian stage schistosomes. Parasitol Today 14: 402–406. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

