HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria
- PMID: 25121750
- PMCID: PMC4294419
- DOI: 10.1016/j.chom.2014.07.003
HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria
Abstract
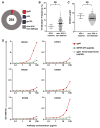
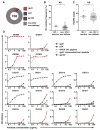
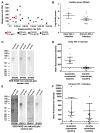
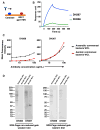
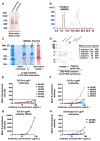
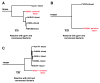
Monoclonal antibodies derived from blood plasma cells of acute HIV-1-infected individuals are predominantly targeted to the HIV Env gp41 and cross-reactive with commensal bacteria. To understand this phenomenon, we examined anti-HIV responses in ileum B cells using recombinant antibody technology and probed their relationship to commensal bacteria. The dominant ileum B cell response was to Env gp41. Remarkably, a majority (82%) of the ileum anti-gp41 antibodies cross-reacted with commensal bacteria, and of those, 43% showed non-HIV-1 antigen polyreactivity. Pyrosequencing revealed shared HIV-1 antibody clonal lineages between ileum and blood. Mutated immunoglobulin G antibodies cross-reactive with both Env gp41 and microbiota could also be isolated from the ileum of HIV-1 uninfected individuals. Thus, the gp41 commensal bacterial antigen cross-reactive antibodies originate in the intestine, and the gp41 Env response in HIV-1 infection can be derived from a preinfection memory B cell pool triggered by commensal bacteria that cross-react with Env.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Alam SM, Scearce RM, Parks RJ, Plonk K, Plonk SG, Sutherland LL, Gorny MK, Zolla-Pazner S, Vanleeuwen S, Moody MA, et al. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J Virol. 2008;82:115–125. - PMC - PubMed
-
- Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–759. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

