Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens
- PMID: 25121752
- PMCID: PMC4157630
- DOI: 10.1016/j.chom.2014.07.002
Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens
Abstract
Inflammasome-mediated host defenses have been extensively studied in innate immune cells. Whether inflammasomes function for innate defense in intestinal epithelial cells, which represent the first line of defense against enteric pathogens, remains unknown. We observed enhanced Salmonella enterica serovar Typhimurium colonization in the intestinal epithelium of caspase-11-deficient mice, but not at systemic sites. In polarized epithelial monolayers, siRNA-mediated depletion of caspase-4, a human ortholog of caspase-11, also led to increased bacterial colonization. Decreased rates of pyroptotic cell death, a host defense mechanism that extrudes S. Typhimurium-infected cells from the polarized epithelium, accounted for increased pathogen burdens. The caspase-4 inflammasome also governs activation of the proinflammatory cytokine, interleukin (IL)-18, in response to intracellular (S. Typhimurium) and extracellular (enteropathogenic Escherichia coli) enteric pathogens, via intracellular LPS sensing. Therefore, an epithelial cell-intrinsic noncanonical inflammasome plays a critical role in antimicrobial defense at the intestinal mucosal surface.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
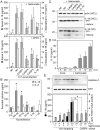

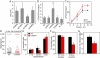
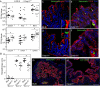
Comment in
-
Getting rid of the bad apple: inflammasome-induced extrusion of Salmonella-infected enterocytes.Cell Host Microbe. 2014 Aug 13;16(2):153-155. doi: 10.1016/j.chom.2014.07.010. Cell Host Microbe. 2014. PMID: 25121744
References
-
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008;8:411–420. - PubMed
-
- Barthel M, Hapfelmeier S, Quintanilla-Martínez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Rüssmann H, Hardt W-D. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003;71:2839–2858. - PMC - PubMed
-
- Bielaszewska M, Rüter C, Kunsmann L, Greune L, Bauwens A, Zhang W, Kuczius T, Kim KS, Mellmann A, Schmidt MA, et al. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 2013;9:e1003797. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

