Metabolome analysis of Drosophila melanogaster during embryogenesis
- PMID: 25121768
- PMCID: PMC4133167
- DOI: 10.1371/journal.pone.0099519
Metabolome analysis of Drosophila melanogaster during embryogenesis
Abstract
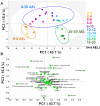
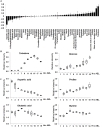
The Drosophila melanogaster embryo has been widely utilized as a model for genetics and developmental biology due to its small size, short generation time, and large brood size. Information on embryonic metabolism during developmental progression is important for further understanding the mechanisms of Drosophila embryogenesis. Therefore, the aim of this study is to assess the changes in embryos' metabolome that occur at different stages of the Drosophila embryonic development. Time course samples of Drosophila embryos were subjected to GC/MS-based metabolome analysis for profiling of low molecular weight hydrophilic metabolites, including sugars, amino acids, and organic acids. The results showed that the metabolic profiles of Drosophila embryo varied during the course of development and there was a strong correlation between the metabolome and different embryonic stages. Using the metabolome information, we were able to establish a prediction model for developmental stages of embryos starting from their high-resolution quantitative metabolite composition. Among the important metabolites revealed from our model, we suggest that different amino acids appear to play distinct roles in different developmental stages and an appropriate balance in trehalose-glucose ratio is crucial to supply the carbohydrate source for the development of Drosophila embryo.
Conflict of interest statement
Figures


References
-
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, et al. (2000) The genome sequence of Drosophila melanogaster . Science 287: 2185–2195. - PubMed
-
- Gilbert S (2000) Early Drosophila Development. Developmental Biology 6th edition.
-
- Tram U, Riggs B, Sullivan W (2001) Cleavage and Gastrulation in Drosophila Embryos. eLS: John Wiley & Sons, Ltd.
-
- Fisher B, Weiszmann R, Frise E, Hammonds A, Tomancak P, et al. (2012) BDGP insitu homepage.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

