Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage
- PMID: 25121790
- PMCID: PMC4237659
- DOI: 10.1227/NEU.0000000000000524
Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage
Abstract
Background: Neonatal germinal matrix hemorrhage/intraventricular hemorrhage is common and often results in hydrocephalus. The pathogenesis of posthemorrhagic hydrocephalus is not fully understood.
Objective: To explore the potential role of hemoglobin and iron released after hemorrhage.
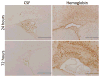
Methods: Artificial cerebrospinal fluid (aCSF), hemoglobin, or iron was injected into the right lateral ventricle of postnatal day-7 Sprague Dawley rats. Ventricle size, heme oxygenase-1 (HO-1) expression, and the presence of iron were evaluated 24 and 72 hours after injection. A subset of animals was treated with an iron chelator (deferoxamine) or vehicle for 24 hours after hemoglobin injection, and ventricle size and cell death were evaluated.
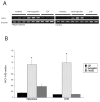
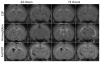
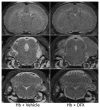
Results: Intraventricular injection of hemoglobin and iron resulted in ventricular enlargement at 24 hours compared with the injection of aCSF. Protoporphyrin IX, the iron-deficient immediate heme precursor, did not result in ventricular enlargement after injection into the ventricle. HO-1, the enzyme that releases iron from heme, was increased in the hippocampus and cortex of hemoglobin-injected animals at 24 hours compared with aCSF-injected controls. Treatment with an iron chelator, deferoxamine, decreased hemoglobin-induced ventricular enlargement and cell death.
Conclusion: Intraventricular injection of hemoglobin and iron can induce hydrocephalus. Treatment with an iron chelator reduced hemoglobin-induced ventricular enlargement. This has implications for the pathogenesis and treatment of posthemorrhagic hydrocephalus.
Abbreviations: aCSF, artificial cerebrospinal fluidDAB, 3,3'-diaminobenzidine-4HClGMH-IVH, germinal matrix hemorrhage/intraventricular hemorrhageHO-1, heme oxygenase-1ICH, intracerebral hemorrhagePBS, phosphate-buffered salineSVZ, subventricular zoneTBST, tris-buffered saline with Tween 20.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

