Lipoprotein-apheresis reduces circulating microparticles in individuals with familial hypercholesterolemia
- PMID: 25121984
- PMCID: PMC4173999
- DOI: 10.1194/jlr.M049726
Lipoprotein-apheresis reduces circulating microparticles in individuals with familial hypercholesterolemia
Abstract
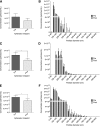
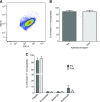
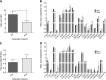
Lipoprotein-apheresis (apheresis) removes LDL-cholesterol in patients with severe dyslipidemia. However, reduction is transient, indicating that the long-term cardiovascular benefits of apheresis may not solely be due to LDL removal. Microparticles (MPs) are submicron vesicles released from the plasma membrane of cells. MPs, particularly platelet-derived MPs, are increasingly being linked to the pathogenesis of many diseases. We aimed to characterize the effect of apheresis on MP size, concentration, cellular origin, and fatty acid concentration in individuals with familial hypercholesterolemia (FH). Plasma and MP samples were collected from 12 individuals with FH undergoing routine apheresis. Tunable resistive pulse sensing (np200) and nanoparticle tracking analysis measured a fall in MP concentration (33 and 15%, respectively; P < 0.05) pre- to post-apheresis. Flow cytometry showed MPs were predominantly annexin V positive and of platelet (CD41) origin both pre- (88.9%) and post-apheresis (88.4%). Fatty acid composition of MPs differed from that of plasma, though apheresis affected a similar profile of fatty acids in both compartments, as measured by GC-flame ionization detection. MP concentration was also shown to positively correlate with thrombin generation potential. In conclusion, we show apheresis nonselectively removes annexin V-positive platelet-derived MPs in individuals with FH. These MPs are potent inducers of coagulation and are elevated in CVD; this reduction in pathological MPs could relate to the long-term benefits of apheresis.
Keywords: exosomes; extracellular vesicles; fatty acids; flow cytometry; low density lipoprotein-apheresis; microvesicles; nanoparticle tracking analysis; phosphatidylserine; tunable resistive pulse sensing.
Copyright © 2014 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Goldstein J. L., Brown M. S. 1979. The LDL receptor locus and the genetics of familial hypercholesterolemia. Annu. Rev. Genet. 13: 259–289. - PubMed
-
- Goldstein J. L., Hobbs H. H., Brown M. S. 2001. Familial hypercholesterolemia. In The Metabolic and Molecular Bases of Inherited Disease. 8th edition. D. Valle, C. R. Scriver, A. L. Beaudet, et al., editors. McGraw-Hill, New York. 2863–2913.
-
- Grundy S. M., Cleeman J. I., Merz C. N., Brewer H. B., Jr, Clark L. T., Hunninghake D. B., Pasternak R. C., Smith S. C., Jr, Stone N. J., et al. ; National Heart, Lung, and Blood Institute. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 110: 227–239. - PubMed
-
- Orsoni A., Villard E. F., Bruckert E., Robillard P., Carrie A., Bonnefont-Rousselot D., Chapman M. J., Dallinga-Thie G. M., Le Goff W., Guerin M. 2012. Impact of LDL apheresis on atheroprotective reverse cholesterol transport pathway in familial hypercholesterolemia. J. Lipid Res. 53: 767–775. - PMC - PubMed
-
- Stoffel W., Borberg H., Greve V. 1981. Application of specific extracorporeal removal of low density lipoprotein in familial hypercholesterolaemia. Lancet. 2: 1005–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

