Vortioxetine disinhibits pyramidal cell function and enhances synaptic plasticity in the rat hippocampus
- PMID: 25122043
- PMCID: PMC4230848
- DOI: 10.1177/0269881114543719
Vortioxetine disinhibits pyramidal cell function and enhances synaptic plasticity in the rat hippocampus
Erratum in
- J Psychopharmacol. 2014 Dec;28(12):1192
Abstract
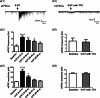
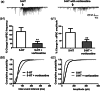
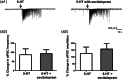
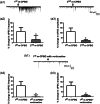
Vortioxetine, a novel antidepressant with multimodal action, is a serotonin (5-HT)3, 5-HT7 and 5-HT1D receptor antagonist, a 5-HT1B receptor partial agonist, a 5-HT1A receptor agonist and a 5-HT transporter (SERT) inhibitor. Vortioxetine has been shown to improve cognitive performance in several preclinical rat models and in patients with major depressive disorder. Here we investigated the mechanistic basis for these effects by studying the effect of vortioxetine on synaptic transmission, long-term potentiation (LTP), a cellular correlate of learning and memory, and theta oscillations in the rat hippocampus and frontal cortex. Vortioxetine was found to prevent the 5-HT-induced increase in inhibitory post-synaptic potentials recorded from CA1 pyramidal cells, most likely by 5-HT3 receptor antagonism. Vortioxetine also enhanced LTP in the CA1 region of the hippocampus. Finally, vortioxetine increased fronto-cortical theta power during active wake in whole animal electroencephalographic recordings. In comparison, the selective SERT inhibitor escitalopram showed no effect on any of these measures. Taken together, our results indicate that vortioxetine can increase pyramidal cell output, which leads to enhanced synaptic plasticity in the hippocampus. Given the central role of the hippocampus in cognition, these findings may provide a cellular correlate to the observed preclinical and clinical cognition-enhancing effects of vortioxetine.
Keywords: 5-hydroxytryptamine 3 receptor; CA1; Serotonin; cognition; electroencephalography; long-term potentiation.
© The Author(s) 2014.
Conflict of interest statement
E Dale, H Zhang, SC Leiser, N Plath and C Sanchez are full-time employees of Lundbeck. The work of Y Xiao, D Lu and C Yang was sponsored by H Lundbeck A/S.
Figures

References
-
- Adell A. (2010) Lu-AA21004, a multimodal serotonergic agent, for the potential treatment of depression and anxiety. IDrugs 13: 900–910. - PubMed
-
- Arai A, Lynch G. (1992) Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res 598: 173–184. - PubMed
-
- Arnsten AF, Lin CH, Van Dyck CH, et al. (1997) The effects of 5-HT3 receptor antagonists on cognitive performance in aged monkeys. Neurobiol Aging 18: 21–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

