Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status
- PMID: 25122430
- PMCID: PMC4224589
- DOI: 10.1097/JTO.0000000000000263
Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status
Abstract
Introduction: In the phase IV, open-label, single-arm study NCT01203917, first-line gefitinib 250 mg/d was effective and well tolerated in Caucasian patients with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer (previously published). Here, we report EGFR mutation analyses of plasma-derived, circulating-free tumor DNA.
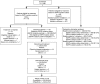
Methods: Mandatory tumor and duplicate plasma (1 and 2) baseline samples were collected (all screened patients; n = 1060). Preplanned, exploratory analyses included EGFR mutation (and subtype) status of tumor versus plasma and between plasma samples. Post hoc, exploratory analyses included efficacy by tumor and plasma EGFR mutation (and subtype) status.
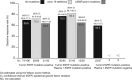
Results: Available baseline tumor samples were 1033 of 1060 (118 positive of 859 mutation status known; mutation frequency, 13.7%). Available plasma 1 samples were 803 of 1060 (82 positive of 784 mutation status known; mutation frequency, 10.5%). Mutation status concordance between 652 matched tumor and plasma 1 samples was 94.3% (95% confidence interval [CI], 92.3-96.0) (comparable for mutation subtypes); test sensitivity was 65.7% (95% CI, 55.8-74.7); and test specificity was 99.8% (95% CI, 99.0-100.0). Twelve patients of unknown tumor mutation status were subsequently identified as plasma mutation-positive. Available plasma 2 samples were 803 of 1060 (65 positive of 224 mutation status-evaluable and -known). Mutation status concordance between 224 matched duplicate plasma 1 and 2 samples was 96.9% (95% CI, 93.7-98.7). Objective response rates are as follows: mutation-positive tumor, 70% (95% CI, 60.5-77.7); mutation-positive tumor and plasma 1, 76.9% (95% CI, 65.4-85.5); and mutation-positive tumor and mutation-negative plasma 1, 59.5% (95% CI, 43.5-73.7). Median progression-free survival (months) was 9.7 (95% CI, 8.5-11.0; 61 events) for mutation-positive tumor and 10.2 (95% CI, 8.5-12.5; 36 events) for mutation-positive tumor and plasma 1.
Conclusion: The high concordance, specificity, and sensitivity demonstrate that EGFR mutation status can be accurately assessed using circulating-free tumor DNA. Although encouraging and suggesting that plasma is a suitable substitute for mutation analysis, tumor tissue should remain the preferred sample type when available.
Trial registration: ClinicalTrials.gov NCT00388206.
Conflict of interest statement
Disclosure: Dr. Douillard has received advisory board and symposia fees from AstraZeneca, Roche, Merck Serono, Amgen, Boehringer Ingelheim, Pfizer, Sanofi-aventis, GlaxoSmithKline, and Bayer Healthcare Pharmaceuticals and has received a research grant from Merck Serono. Dr. Cole, Ms. McWalter, Dr. Walker, Mr. Dearden, Mr. Webster, Dr. Milenkova, and Dr. McCormack are employees of AstraZeneca and hold shares in AstraZeneca. All other authors declare no conflict of interest.
Figures

References
-
- Vallières E, Peters S, Van Houtte P, Dalal P, Lim E. Therapeutic advances in non-small cell lung cancer. Thorax. 2012;67:1097–1101. - PubMed
-
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–859. - PMC - PubMed
-
- Health and Social Care Information Centre NLCA. National Lung Cancer Audit Report 2012. Available at: http://www.hqip.org.uk/assets/NCAPOP-Library/NCAPOP-2012–13/Lung-Cancer-.... Accessed September 27, 2013.
-
- Goto K, Ichinose Y, Ohe Y, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol. 2012;7:115–121. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

