Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates
- PMID: 25122523
- PMCID: PMC4372899
- DOI: 10.1038/nprot.2014.143
Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates
Abstract
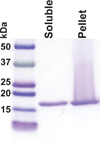
This protocol describes a primary neuronal model of formation of α-synuclein (α-syn) aggregates that recapitulate features of the Lewy bodies and Lewy neurites found in Parkinson's disease brains and other synucleinopathies. This model allows investigation of aggregate formation, their impact on neuron function, and development of therapeutics. Addition of preformed fibrils (PFFs) synthesized from recombinant α-syn to neurons seeds the recruitment of endogenous α-syn into aggregates characterized by detergent insolubility and hyperphosphorylation. Aggregate formation follows a lag phase of 2-3 d, followed by formation in axons by days 4-7, spread to somatodendritic compartments by days 7-10 and neuron death ~14 d after PFF addition. Here we provide methods and highlight the crucial steps for PFF formation, PFF addition to cultured hippocampal neurons and confirmation of aggregate formation. Neurons derived from various brain regions from nontransgenic and genetically engineered mice and rats can be used, allowing interrogation of the effect of specific genes on aggregate formation.
Figures





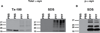
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

