Lymph node fibroblastic reticular cell transplants show robust therapeutic efficacy in high-mortality murine sepsis
- PMID: 25122637
- PMCID: PMC4415170
- DOI: 10.1126/scitranslmed.3009377
Lymph node fibroblastic reticular cell transplants show robust therapeutic efficacy in high-mortality murine sepsis
Abstract
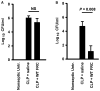
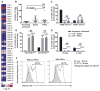
Sepsis is an aggressive inflammatory syndrome and a global health burden estimated to kill 7.3 million people annually. Single-target molecular therapies have not addressed the multiple disease pathways triggered by septic injury. Cell therapies might offer a broader set of mechanisms of action that benefit complex, multifocal disease processes. We describe a population of immune-specialized myofibroblasts derived from lymph node tissue, termed fibroblastic reticular cells (FRCs). Because FRCs have an immunoregulatory function in lymph nodes, we hypothesized that ex vivo-expanded FRCs would control inflammation when administered therapeutically. Indeed, a single injection of ex vivo-expanded allogeneic FRCs reduced mortality in mouse models of sepsis when administered at early or late time points after septic onset. Mice treated with FRCs exhibited lower local and systemic concentrations of proinflammatory cytokines and reduced bacteremia. When administered 4 hours after induction of lipopolysaccharide endotoxemia, or cecal ligation and puncture (CLP) sepsis in mice, FRCs reduced deaths by at least 70%. When administered late in disease (16 hours after CLP), FRCs still conveyed a robust survival advantage (44% survival compared to 0% for controls). FRC therapy was dependent on the metabolic activity of nitric oxide synthase 2 (NOS2) as the primary molecular mechanism of drug action in the mice. Together, these data describe a new anti-inflammatory cell type and provide preclinical evidence for therapeutic efficacy in severe sepsis that warrants further translational study.
Copyright © 2014, American Association for the Advancement of Science.
Conflict of interest statement
Figures


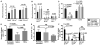

References
-
- Carney DE, Matsushima K, Frankel HL. Treatment of sepsis in the surgical intensive care unit. Isr Med Assoc J. 2011;13:694–699. - PubMed
-
- Daniels R. Surviving the first hours in sepsis: Getting the basics right (an intensivist’s perspective) J Antimicrob Chemother. 2011;66(Suppl 2):ii11–ii23. - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy MS. Inpatient Care for Septicemia or Sepsis: A Challenge for Patients and Hospitals. National Center for Health Statistics; Hyattsville, MD: 2011. - PubMed
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v.2.0. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. http://globocan.iarc.fr.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

