Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy
- PMID: 25122679
- PMCID: PMC4191793
- DOI: 10.1073/pnas.1404171111
Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy
Abstract
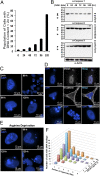
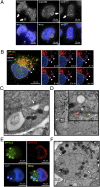
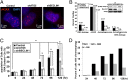
Autophagy is the principal catabolic prosurvival pathway during nutritional starvation. However, excessive autophagy could be cytotoxic, contributing to cell death, but its mechanism remains elusive. Arginine starvation has emerged as a potential therapy for several types of cancers, owing to their tumor-selective deficiency of the arginine metabolism. We demonstrated here that arginine depletion by arginine deiminase induces a cytotoxic autophagy in argininosuccinate synthetase (ASS1)-deficient prostate cancer cells. Advanced microscopic analyses of arginine-deprived dying cells revealed a novel phenotype with giant autophagosome formation, nucleus membrane rupture, and histone-associated DNA leakage encaptured by autophagosomes, which we shall refer to as chromatin autophagy, or chromatophagy. In addition, nuclear inner membrane (lamin A/C) underwent localized rearrangement and outer membrane (NUP98) partially fused with autophagosome membrane. Further analysis showed that prolonged arginine depletion impaired mitochondrial oxidative phosphorylation function and depolarized mitochondrial membrane potential. Thus, reactive oxygen species (ROS) production significantly increased in both cytosolic and mitochondrial fractions, presumably leading to DNA damage accumulation. Addition of ROS scavenger N-acetyl cysteine or knockdown of ATG5 or BECLIN1 attenuated the chromatophagy phenotype. Our data uncover an atypical autophagy-related death pathway and suggest that mitochondrial damage is central to linking arginine starvation and chromatophagy in two distinct cellular compartments.
Keywords: ADI-PEG20; arginine auxotrophy; cancer therapy; metabolic stress; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Arginine deprivation and autophagic cell death in cancer.Proc Natl Acad Sci U S A. 2014 Sep 30;111(39):14015-6. doi: 10.1073/pnas.1416560111. Epub 2014 Sep 16. Proc Natl Acad Sci U S A. 2014. PMID: 25228774 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

