Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats
- PMID: 25122682
- PMCID: PMC4156750
- DOI: 10.1073/pnas.1406768111
Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats
Abstract
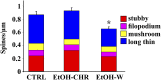
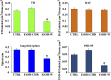
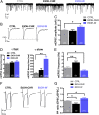
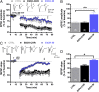
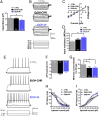
Alcoholism involves long-term cognitive deficits, including memory impairment, resulting in substantial cost to society. Neuronal refinement and stabilization are hypothesized to confer resilience to poor decision making and addictive-like behaviors, such as excessive ethanol drinking and dependence. Accordingly, structural abnormalities are likely to contribute to synaptic dysfunctions that occur from suddenly ceasing the use of alcohol after chronic ingestion. Here we show that ethanol-dependent rats display a loss of dendritic spines in medium spiny neurons of the nucleus accumbens (Nacc) shell, accompanied by a reduction of tyrosine hydroxylase immunostaining and postsynaptic density 95-positive elements. Further analysis indicates that "long thin" but not "mushroom" spines are selectively affected. In addition, patch-clamp experiments from Nacc slices reveal that long-term depression (LTD) formation is hampered, with parallel changes in field potential recordings and reductions in NMDA-mediated synaptic currents. These changes are restricted to the withdrawal phase of ethanol dependence, suggesting their relevance in the genesis of signs and/or symptoms affecting ethanol withdrawal and thus the whole addictive cycle. Overall, these results highlight the key role of dynamic alterations in dendritic spines and their presynaptic afferents in the evolution of alcohol dependence. Furthermore, they suggest that the selective loss of long thin spines together with a reduced NMDA receptor function may affect learning. Disruption of this LTD could contribute to the rigid emotional and motivational state observed in alcohol dependence.
Keywords: Golgi; dopamine; glutamate; synaptic plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516–524. - PubMed
-
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: Hypodopaminergic state. Int Rev Neurobiol. 2005;63:101–154. - PubMed
-
- Kalivas PW, Brady K. Getting to the core of addiction: Hatching the addiction egg. Nat Med. 2012;18(4):502–503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

