Increased intrinsic excitability of muscle vasoconstrictor preganglionic neurons may contribute to the elevated sympathetic activity in hypertensive rats
- PMID: 25122704
- PMCID: PMC4254885
- DOI: 10.1152/jn.00350.2014
Increased intrinsic excitability of muscle vasoconstrictor preganglionic neurons may contribute to the elevated sympathetic activity in hypertensive rats
Abstract
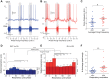
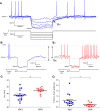
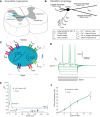
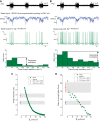
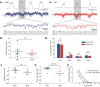
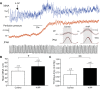
Hypertension is associated with pathologically increased sympathetic drive to the vasculature. This has been attributed to increased excitatory drive to sympathetic preganglionic neurons (SPN) from brainstem cardiovascular control centers. However, there is also evidence supporting increased intrinsic excitability of SPN. To test this hypothesis, we made whole cell recordings of muscle vasoconstrictor-like (MVClike) SPN in the working-heart brainstem preparation of spontaneously hypertensive (SH) and normotensive Wistar-Kyoto (WKY) rats. The MVClike SPN have a higher spontaneous firing frequency in the SH rat (3.85 ± 0.4 vs. 2.44 ± 0.4 Hz in WKY; P = 0.011) with greater respiratory modulation of their activity. The action potentials of SH SPN had smaller, shorter afterhyperpolarizations (AHPs) and showed diminished transient rectification indicating suppression of an A-type potassium conductance (IA). We developed mathematical models of the SPN to establish if changes in their intrinsic properties in SH rats could account for their altered firing. Reduction of the maximal conductance density of IA by 15-30% changed the excitability and output of the model from the WKY to a SH profile, with increased firing frequency, amplified respiratory modulation, and smaller AHPs. This change in output is predominantly a consequence of altered synaptic integration. Consistent with these in silico predictions, we found that intrathecal 4-aminopyridine (4-AP) increased sympathetic nerve activity, elevated perfusion pressure, and augmented Traube-Hering waves. Our findings indicate that IA acts as a powerful filter on incoming synaptic drive to SPN and that its diminution in the SH rat is potentially sufficient to account for the increased sympathetic output underlying hypertension.
Keywords: hypertension; sympathetic preganglionic; transient rectification; vasomotor tone.
Copyright © 2014 the American Physiological Society.
Figures






References
-
- Belugin S, Mifflin S. Transient voltage-dependent potassium currents are reduced in NTS neurons isolated from renal wrap hypertensive rats. J Neurophysiol 94: 3849–3859, 2005. - PubMed
-
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science 305: 532–535, 2004. - PubMed
-
- Bordey A, Feltz P, Trouslard J. Kinetics of A-currents in sympathetic preganglionic neurones and glial cells. Neuroreport 7: 37–40, 1995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

