Sensory-evoked and spontaneous gamma and spindle bursts in neonatal rat motor cortex
- PMID: 25122889
- PMCID: PMC6705262
- DOI: 10.1523/JNEUROSCI.4539-13.2014
Sensory-evoked and spontaneous gamma and spindle bursts in neonatal rat motor cortex
Abstract
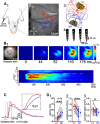
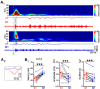
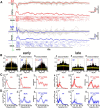
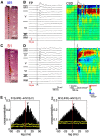
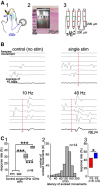
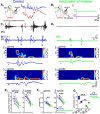
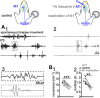
Self-generated neuronal activity originating from subcortical regions drives early spontaneous motor activity, which is a hallmark of the developing sensorimotor system. However, the neural activity patterns and role of primary motor cortex (M1) in these early movements are still unknown. Combining voltage-sensitive dye imaging (VSDI) with simultaneous extracellular multielectrode recordings in postnatal day 3 (P3)-P5 rat primary somatosensory cortex (S1) and M1 in vivo, we observed that tactile forepaw stimulation induced spindle bursts in S1 and gamma and spindle bursts in M1. Approximately 40% of the spontaneous gamma and spindle bursts in M1 were driven by early motor activity, whereas 23.7% of the M1 bursts triggered forepaw movements. Approximately 35% of the M1 bursts were uncorrelated to movements and these bursts had significantly fewer spikes and shorter burst duration. Focal electrical stimulation of layer V neurons in M1 mimicking physiologically relevant 40 Hz gamma or 10 Hz spindle burst activity reliably elicited forepaw movements. We conclude that M1 is already involved in somatosensory information processing during early development. M1 is mainly activated by tactile stimuli triggered by preceding spontaneous movements, which reach M1 via S1. Only a fraction of M1 activity transients trigger motor responses directly. We suggest that both spontaneously occurring and sensory-evoked gamma and spindle bursts in M1 contribute to the maturation of corticospinal and sensorimotor networks required for the refinement of sensorimotor coordination.
Keywords: development; in vivo; motor; neocortex; rat; somatosensory.
Copyright © 2014 the authors 0270-6474/14/3410870-14$15.00/0.
Figures



References
-
- Berger T, Borgdorff A, Crochet S, Neubauer FB, Lefort S, Fauvet B, Ferezou I, Carleton A, Lüscher HR, Petersen CC. Combined voltage and calcium epifluorescence Imaging in vitro and in vivo reveals subthreshold and suprathreshold dynamics of mouse barrel cortex. J Neurophysiol. 2007;97:3751–3762. doi: 10.1152/jn.01178.2006. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
