High-NaCl perception in Drosophila melanogaster
- PMID: 25122890
- PMCID: PMC6705259
- DOI: 10.1523/JNEUROSCI.4795-13.2014
High-NaCl perception in Drosophila melanogaster
Abstract
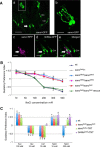
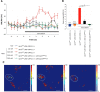
Salt is a fundamental nutrient that is required for many physiological processes, including electrolyte homeostasis and neuronal activity. In mammals and Drosophila, the detection of NaCl induces two different behaviors: low-salt concentrations provide an attractive stimulus, whereas high-salt concentrations are avoided. We identified the gene called serrano (sano) as being expressed in the sensory organs of Drosophila larvae. A transgenic reporter line showed that sano was coexpressed with Gr66a in a subset of gustatory neurons in the terminal organ of third-instar larvae. The disruption of sano gene expression in gustatory neurons led to the specific loss of high-salt concentration avoidance in larvae, whereas the detection of other attractive or aversive substances was unaffected. Moreover, using a cellular marker sensitive to calcium levels, Sano function was shown to be required for neuronal activity in response to high-salt concentrations. In these neurons, the loss of the DEG/ENaC channel PPK19 function also eliminated the cellular response to high-salt concentrations. Our study revealed that PPK19 and Sano are required in the neurons of the larval gustatory organs for the detection of high-salt concentrations.
Keywords: Drosophila melanogaster; behavior; chemosensory system; larva; salt; taste.
Copyright © 2014 the authors 0270-6474/14/3410884-08$15.00/0.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
