Ribosome profiling reveals a cell-type-specific translational landscape in brain tumors
- PMID: 25122893
- PMCID: PMC4131009
- DOI: 10.1523/JNEUROSCI.0084-14.2014
Ribosome profiling reveals a cell-type-specific translational landscape in brain tumors
Abstract
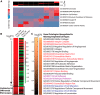
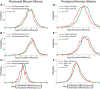
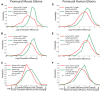
Glioma growth is driven by signaling that ultimately regulates protein synthesis. Gliomas are also complex at the cellular level and involve multiple cell types, including transformed and reactive cells in the brain tumor microenvironment. The distinct functions of the various cell types likely lead to different requirements and regulatory paradigms for protein synthesis. Proneural gliomas can arise from transformation of glial progenitors that are driven to proliferate via mitogenic signaling that affects translation. To investigate translational regulation in this system, we developed a RiboTag glioma mouse model that enables cell-type-specific, genome-wide ribosome profiling of tumor tissue. Infecting glial progenitors with Cre-recombinant retrovirus simultaneously activates expression of tagged ribosomes and delivers a tumor-initiating mutation. Remarkably, we find that although genes specific to transformed cells are highly translated, their translation efficiencies are low compared with normal brain. Ribosome positioning reveals sequence-dependent regulation of ribosomal activity in 5'-leaders upstream of annotated start codons, leading to differential translation in glioma compared with normal brain. Additionally, although transformed cells express a proneural signature, untransformed tumor-associated cells, including reactive astrocytes and microglia, express a mesenchymal signature. Finally, we observe the same phenomena in human disease by combining ribosome profiling of human proneural tumor and non-neoplastic brain tissue with computational deconvolution to assess cell-type-specific translational regulation.
Keywords: cell-type-specific expression; glioblastoma; glioma; ribosome profiling; translational regulation.
Copyright © 2014 the authors 0270-6474/14/3410924-13$15.00/0.
Figures




Similar articles
-
Identification of global alteration of translational regulation in glioma in vivo.PLoS One. 2012;7(10):e46965. doi: 10.1371/journal.pone.0046965. Epub 2012 Oct 3. PLoS One. 2012. PMID: 23056544 Free PMC article.
-
Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process.Proc Natl Acad Sci U S A. 2014 Oct 7;111(40):E4214-23. doi: 10.1073/pnas.1414389111. Epub 2014 Sep 22. Proc Natl Acad Sci U S A. 2014. PMID: 25246577 Free PMC article.
-
The cell of origin dictates the temporal course of neurofibromatosis-1 (Nf1) low-grade glioma formation.Oncotarget. 2017 Jul 18;8(29):47206-47215. doi: 10.18632/oncotarget.17589. Oncotarget. 2017. PMID: 28525381 Free PMC article.
-
Recent insights into PDGF-induced gliomagenesis.Brain Pathol. 2010 May;20(3):527-38. doi: 10.1111/j.1750-3639.2009.00335.x. Epub 2009 Sep 25. Brain Pathol. 2010. PMID: 19832839 Free PMC article. Review.
-
Genome-wide translation patterns in gliomas: An integrative view.Cell Signal. 2021 Mar;79:109883. doi: 10.1016/j.cellsig.2020.109883. Epub 2020 Dec 13. Cell Signal. 2021. PMID: 33321181 Review.
Cited by
-
RiboTag is a flexible tool for measuring the translational state of targeted cells in heterogeneous cell cultures.Biotechniques. 2015 Jun 1;58(6):308-17. doi: 10.2144/000114299. eCollection 2015 Jun. Biotechniques. 2015. PMID: 26054767 Free PMC article.
-
Defects in translation-dependent quality control pathways lead to convergent molecular and neurodevelopmental pathology.Elife. 2021 Apr 26;10:e66904. doi: 10.7554/eLife.66904. Elife. 2021. PMID: 33899734 Free PMC article.
-
Relevance of Translation Initiation in Diffuse Glioma Biology and its Therapeutic Potential.Cells. 2019 Nov 29;8(12):1542. doi: 10.3390/cells8121542. Cells. 2019. PMID: 31795417 Free PMC article. Review.
-
How Messenger RNA and Nascent Chain Sequences Regulate Translation Elongation.Annu Rev Biochem. 2018 Jun 20;87:421-449. doi: 10.1146/annurev-biochem-060815-014818. Annu Rev Biochem. 2018. PMID: 29925264 Free PMC article. Review.
-
Comparative survey of the relative impact of mRNA features on local ribosome profiling read density.Nat Commun. 2016 Oct 4;7:12915. doi: 10.1038/ncomms12915. Nat Commun. 2016. PMID: 27698342 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
