Network structure within the cerebellar input layer enables lossless sparse encoding
- PMID: 25123311
- PMCID: PMC4148198
- DOI: 10.1016/j.neuron.2014.07.020
Network structure within the cerebellar input layer enables lossless sparse encoding
Abstract
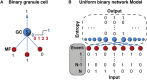
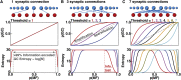
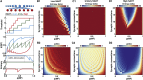
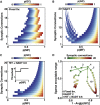
The synaptic connectivity within neuronal networks is thought to determine the information processing they perform, yet network structure-function relationships remain poorly understood. By combining quantitative anatomy of the cerebellar input layer and information theoretic analysis of network models, we investigated how synaptic connectivity affects information transmission and processing. Simplified binary models revealed that the synaptic connectivity within feedforward networks determines the trade-off between information transmission and sparse encoding. Networks with few synaptic connections per neuron and network-activity-dependent threshold were optimal for lossless sparse encoding over the widest range of input activities. Biologically detailed spiking network models with experimentally constrained synaptic conductances and inhibition confirmed our analytical predictions. Our results establish that the synaptic connectivity within the cerebellar input layer enables efficient lossless sparse encoding. Moreover, they provide a functional explanation for why granule cells have approximately four dendrites, a feature that has been evolutionarily conserved since the appearance of fish.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Albus J.S. A theory of cerebellar function. Math. Biosci. 1971;10:25–61.
-
- Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Brickley S.G., Revilla V., Cull-Candy S.G., Wisden W., Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

