Implantable impedance plethysmography
- PMID: 25123467
- PMCID: PMC4179001
- DOI: 10.3390/s140814858
Implantable impedance plethysmography
Abstract
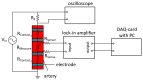
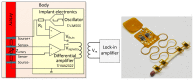
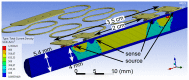
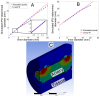
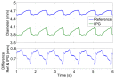
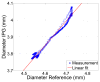
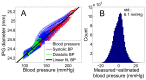
We demonstrate by theory, as well as by ex vivo and in vivo measurements that impedance plethysmography, applied extravascularly directly on large arteries, is a viable method for monitoring various cardiovascular parameters, such as blood pressure, with high accuracy. The sensor is designed as an implant to monitor cardiac events and arteriosclerotic progression over the long term.
Figures





References
-
- World Health Organization . Global Health Risk—Mortality and Burdan of Disease Attributable to Selected Major Risk. World Health Organization; Geneva, Switzerland: 2009.
-
- OBrien E., Asmar R., Beilin L., Verdecchia P. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J. Hypertens. 2003;21:821–848. - PubMed
-
- Vij R., Peixoto A. Management of nocturnal hypertension. Expert Rev. Cardiovasc. Ther. 2009;7:607–618. - PubMed
-
- Heinen-Kammerer T., Wiosna W., Nelles S., Rychlik R. Health Technology Assessment-Bericht 30: Monitoring von Herzfunktionen mit Telemetrie. Heinen-Kammerer; Cologne, Germany: 2006.
-
- Boriani G., Diemberger I., Martignani C., Biffi M., Valzania C., Bertini M., Domenichini G., Saporito D., Ziacchi M., Branzi A. Telecardiology and Remote Monitoring of Implanted Electrical Devices: The Potential for Fresh Clinical Care Perspectives. J. Gen. Intern. Med. 2008;23:73–77. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

