A comprehensive survey of non-canonical splice sites in the human transcriptome
- PMID: 25123659
- PMCID: PMC4176328
- DOI: 10.1093/nar/gku744
A comprehensive survey of non-canonical splice sites in the human transcriptome
Abstract
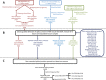
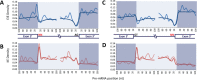
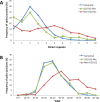
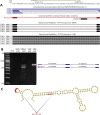
We uncovered the diversity of non-canonical splice sites at the human transcriptome using deep transcriptome profiling. We mapped a total of 3.7 billion human RNA-seq reads and developed a set of stringent filters to avoid false non-canonical splice site detections. We identified 184 splice sites with non-canonical dinucleotides and U2/U12-like consensus sequences. We selected 10 of the herein identified U2/U12-like non-canonical splice site events and successfully validated 9 of them via reverse transcriptase-polymerase chain reaction and Sanger sequencing. Analyses of the 184 U2/U12-like non-canonical splice sites indicate that 51% of them are not annotated in GENCODE. In addition, 28% of them are conserved in mouse and 76% are involved in alternative splicing events, some of them with tissue-specific alternative splicing patterns. Interestingly, our analysis identified some U2/U12-like non-canonical splice sites that are converted into canonical splice sites by RNA A-to-I editing. Moreover, the U2/U12-like non-canonical splice sites have a differential distribution of splicing regulatory sequences, which may contribute to their recognition and regulation. Our analysis provides a high-confidence group of U2/U12-like non-canonical splice sites, which exhibit distinctive features among the total human splice sites.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Aebi M., Hornig H., Padgett R.A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986;47:555–565. - PubMed
-
- Lamond A.I., Konarska M.M., Sharp P.A. A mutational analysis of spliceosome assembly: evidence for splice site collaboration during spliceosome formation. Genes Dev. 1987;1:532–543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

