DEAD-box RNA helicase domains exhibit a continuum between complete functional independence and high thermodynamic coupling in nucleotide and RNA duplex recognition
- PMID: 25123660
- PMCID: PMC4176333
- DOI: 10.1093/nar/gku747
DEAD-box RNA helicase domains exhibit a continuum between complete functional independence and high thermodynamic coupling in nucleotide and RNA duplex recognition
Abstract
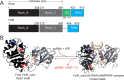
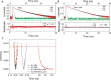
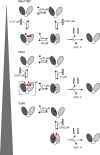
DEAD-box helicases catalyze the non-processive unwinding of double-stranded RNA (dsRNA) at the expense of adenosine triphosphate (ATP) hydrolysis. Nucleotide and RNA binding and unwinding are mediated by the RecA domains of the helicase core, but their cooperation in these processes remains poorly understood. We therefore investigated dsRNA and nucleotide binding by the helicase cores and the isolated N- and C-terminal RecA domains (RecA_N, RecA_C) of the DEAD-box proteins Hera and YxiN by steady-state and time-resolved fluorescence methods. Both helicases bind nucleotides predominantly via RecA_N, in agreement with previous studies on Mss116, and with a universal, modular function of RecA_N in nucleotide recognition. In contrast, dsRNA recognition is different: Hera interacts with dsRNA in the absence of nucleotide, involving both RecA domains, whereas for YxiN neither RecA_N nor RecA_C binds dsRNA, and the complete core only interacts with dsRNA after nucleotide has been bound. DEAD-box proteins thus cover a continuum from complete functional independence of their domains, exemplified by Mss116, to various degrees of inter-domain cooperation in dsRNA binding. The different degrees of domain communication and of thermodynamic linkage between dsRNA and nucleotide binding have important implications on the mechanism of dsRNA unwinding, and may help direct RNA helicases to their respective cellular processes.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Steimer L., Klostermeier D. RNA helicases in infection and disease. RNA Biol. 2012;9:751–771. - PubMed
-
- Kossen K., Karginov F.V., Uhlenbeck O.C. The carboxy-terminal domain of the DExDH protein YxiN is sufficient to confer specificity for 23S rRNA. J. Mol. Biol. 2002;324:625–636. - PubMed
-
- Karginov F.V., Caruthers J.M., Hu Y., McKay D.B., Uhlenbeck O.C. YxiN is a modular protein combining a DEx(D/H) core and a specific RNA-binding domain. J. Biol. Chem. 2005;280:35499–35505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

