Proteomic analysis of the Plasmodium male gamete reveals the key role for glycolysis in flagellar motility
- PMID: 25124718
- PMCID: PMC4150949
- DOI: 10.1186/1475-2875-13-315
Proteomic analysis of the Plasmodium male gamete reveals the key role for glycolysis in flagellar motility
Abstract
Background: Gametogenesis and fertilization play crucial roles in malaria transmission. While male gametes are thought to be amongst the simplest eukaryotic cells and are proven targets of transmission blocking immunity, little is known about their molecular organization. For example, the pathway of energy metabolism that power motility, a feature that facilitates gamete encounter and fertilization, is unknown.
Methods: Plasmodium berghei microgametes were purified and analysed by whole-cell proteomic analysis for the first time. Data are available via ProteomeXchange with identifier PXD001163.
Results: 615 proteins were recovered, they included all male gamete proteins described thus far. Amongst them were the 11 enzymes of the glycolytic pathway. The hexose transporter was localized to the gamete plasma membrane and it was shown that microgamete motility can be suppressed effectively by inhibitors of this transporter and of the glycolytic pathway.
Conclusions: This study describes the first whole-cell proteomic analysis of the malaria male gamete. It identifies glycolysis as the likely exclusive source of energy for flagellar beat, and provides new insights in original features of Plasmodium flagellar organization.
Figures
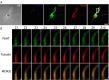
 or absence
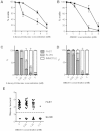
or absence  of excess glucose (10 mM). (C, D) Patterns of motility were further categorized into fast beat, slow beat and immotile. (E) Values of flagella wave frequencies were measured with different concentrations of CM3361. Frequencies of waves were not altered by the presence of the inhibitor.
of excess glucose (10 mM). (C, D) Patterns of motility were further categorized into fast beat, slow beat and immotile. (E) Values of flagella wave frequencies were measured with different concentrations of CM3361. Frequencies of waves were not altered by the presence of the inhibitor.
Similar articles
-
Dynamic molecular events associated to Plasmodium berghei gametogenesis through proteomic approach.J Proteomics. 2018 May 30;180:88-98. doi: 10.1016/j.jprot.2017.11.009. Epub 2017 Nov 15. J Proteomics. 2018. PMID: 29155091
-
The Armadillo repeat protein PF16 is essential for flagellar structure and function in Plasmodium male gametes.PLoS One. 2010 Sep 23;5(9):e12901. doi: 10.1371/journal.pone.0012901. PLoS One. 2010. PMID: 20886115 Free PMC article.
-
Vital role for Plasmodium berghei Kinesin8B in axoneme assembly during male gamete formation and mosquito transmission.Cell Microbiol. 2020 Mar;22(3):e13121. doi: 10.1111/cmi.13121. Epub 2019 Dec 12. Cell Microbiol. 2020. PMID: 31634979
-
Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro.Vaccine. 2009 Aug 20;27(38):5187-94. doi: 10.1016/j.vaccine.2009.06.069. Epub 2009 Jul 9. Vaccine. 2009. PMID: 19596419 Review.
-
Fertilization is a novel attacking site for the transmission blocking of malaria parasites.Acta Trop. 2010 Jun;114(3):157-61. doi: 10.1016/j.actatropica.2009.08.005. Epub 2009 Aug 8. Acta Trop. 2010. PMID: 19665985 Review.
Cited by
-
Multiple short windows of calcium-dependent protein kinase 4 activity coordinate distinct cell cycle events during Plasmodium gametogenesis.Elife. 2017 May 8;6:e26524. doi: 10.7554/eLife.26524. Elife. 2017. PMID: 28481199 Free PMC article.
-
The cell biology of malaria infection of mosquito: advances and opportunities.Cell Microbiol. 2015 Apr;17(4):451-66. doi: 10.1111/cmi.12413. Epub 2015 Feb 4. Cell Microbiol. 2015. PMID: 25557077 Free PMC article. Review.
-
Clinicopathological Analysis and Multipronged Quantitative Proteomics Reveal Oxidative Stress and Cytoskeletal Proteins as Possible Markers for Severe Vivax Malaria.Sci Rep. 2016 Apr 19;6:24557. doi: 10.1038/srep24557. Sci Rep. 2016. PMID: 27090372 Free PMC article.
-
An overview of the Plasmodium falciparum hexose transporter and its therapeutic interventions.Proteins. 2022 Oct;90(10):1766-1778. doi: 10.1002/prot.26351. Epub 2022 May 6. Proteins. 2022. PMID: 35445447 Free PMC article. Review.
-
Genome-wide functional analysis reveals key roles for kinesins in the mammalian and mosquito stages of the malaria parasite life cycle.PLoS Biol. 2022 Jul 28;20(7):e3001704. doi: 10.1371/journal.pbio.3001704. eCollection 2022 Jul. PLoS Biol. 2022. PMID: 35900985 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

