Vector platforms for gene therapy of inherited retinopathies
- PMID: 25124745
- PMCID: PMC4241499
- DOI: 10.1016/j.preteyeres.2014.08.001
Vector platforms for gene therapy of inherited retinopathies
Abstract
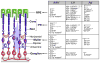
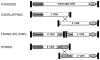
Inherited retinopathies (IR) are common untreatable blinding conditions. Most of them are inherited as monogenic disorders, due to mutations in genes expressed in retinal photoreceptors (PR) and in retinal pigment epithelium (RPE). The retina's compatibility with gene transfer has made transduction of different retinal cell layers in small and large animal models via viral and non-viral vectors possible. The ongoing identification of novel viruses as well as modifications of existing ones based either on rational design or directed evolution have generated vector variants with improved transduction properties. Dozens of promising proofs of concept have been obtained in IR animal models with both viral and non-viral vectors, and some of them have been relayed to clinical trials. To date, recombinant vectors based on the adeno-associated virus (AAV) represent the most promising tool for retinal gene therapy, given their ability to efficiently deliver therapeutic genes to both PR and RPE and their excellent safety and efficacy profiles in humans. However, AAVs' limited cargo capacity has prevented application of the viral vector to treatments requiring transfer of genes with a coding sequence larger than 5 kb. Vectors with larger capacity, i.e. nanoparticles, adenoviral and lentiviral vectors are being exploited for gene transfer to the retina in animal models and, more recently, in humans. This review focuses on the available platforms for retinal gene therapy to fight inherited blindness, highlights their main strengths and examines the efforts to overcome some of their limitations.
Keywords: Adeno-associated virus; Adenovirus; Gene therapy; Inherited retinopathies; Lentivirus; Non-viral vectors.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
References
-
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. - PubMed
-
- Akimoto M, Miyatake S, Kogishi J, Hangai M, Okazaki K, Takahashi JC, Saiki M, Iwaki M, Honda Y. Adenovirally expressed basic fibroblast growth factor rescues photoreceptor cells in RCS rats. Invest Ophthalmol Vis Sci. 1999;40:273–279. - PubMed
-
- Ali RR, Reichel MB, De Alwis M, Kanuga N, Kinnon C, Levinsky RJ, Hunt DM, Bhattacharya SS, Thrasher AJ. Adeno-associated virus gene transfer to mouse retina. Hum Gene Ther. 1998;9:81–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

