Hydrogen peroxide primes heart regeneration with a derepression mechanism
- PMID: 25124925
- PMCID: PMC4152734
- DOI: 10.1038/cr.2014.108
Hydrogen peroxide primes heart regeneration with a derepression mechanism
Abstract
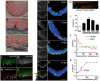
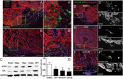
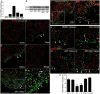
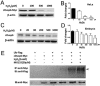
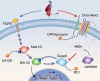
While the adult human heart has very limited regenerative potential, the adult zebrafish heart can fully regenerate after 20% ventricular resection. Although previous reports suggest that developmental signaling pathways such as FGF and PDGF are reused in adult heart regeneration, the underlying intracellular mechanisms remain largely unknown. Here we show that H2O2 acts as a novel epicardial and myocardial signal to prime the heart for regeneration in adult zebrafish. Live imaging of intact hearts revealed highly localized H2O2 (~30 μM) production in the epicardium and adjacent compact myocardium at the resection site. Decreasing H2O2 formation with the Duox inhibitors diphenyleneiodonium (DPI) or apocynin, or scavenging H2O2 by catalase overexpression markedly impaired cardiac regeneration while exogenous H2O2 rescued the inhibitory effects of DPI on cardiac regeneration, indicating that H2O2 is an essential and sufficient signal in this process. Mechanistically, elevated H2O2 destabilized the redox-sensitive phosphatase Dusp6 and hence increased the phosphorylation of Erk1/2. The Dusp6 inhibitor BCI achieved similar pro-regenerative effects while transgenic overexpression of dusp6 impaired cardiac regeneration. H2O2 plays a dual role in recruiting immune cells and promoting heart regeneration through two relatively independent pathways. We conclude that H2O2 potentially generated from Duox/Nox2 promotes heart regeneration in zebrafish by unleashing MAP kinase signaling through a derepression mechanism involving Dusp6.
Figures

References
-
- Xiong JW, Chang NN. Recent advances in heart regeneration. Birth Defects Res C Embryo Today. 2013;99:160–169. - PubMed
-
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. - PubMed
-
- Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 2011;138:1663–1674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

