Getting folded: chaperone proteins in muscle development, maintenance and disease
- PMID: 25125177
- PMCID: PMC4135391
- DOI: 10.1002/ar.22980
Getting folded: chaperone proteins in muscle development, maintenance and disease
Abstract
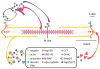
Chaperone proteins are critical for protein folding and stability, and hence are necessary for normal cellular organization and function. Recent studies have begun to interrogate the role of this specialized class of proteins in muscle biology. During development, chaperone-mediated folding of client proteins enables their integration into nascent functional sarcomeres. In addition to assisting with muscle differentiation, chaperones play a key role in the maintenance of muscle tissues. Furthermore, disruption of the chaperone network can result in neuromuscular disease. In this review, we discuss how chaperones are involved in myofibrillogenesis, sarcomere maintenance, and muscle disorders. We also consider the possibilities of therapeutically targeting chaperones to treat muscle disease.
Keywords: chaperones; contractile proteins; myofibril; protein folding; sarcomere.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest: The authors have no financial or other conflicts of interest to disclose.
Figures


References
-
- Agarraberes FA, Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114:2491–2499. - PubMed
-
- Andley UP, Mathur S, Griest TA, Petrash JM. Cloning, expression, and chaperone-like activity of human alphaA-crystallin. J Biol Chem. 1996;271:31973–31980. - PubMed
-
- Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Fürst DO, Saftig P, Saint R, Fleischmann BK, et al. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol. 2010;20:143–148. - PubMed
-
- Atomi Y, Yamada S, Strohman R, Nonomura Y. Alpha B-crystallin in skeletal muscle: purification and localization. J Biochem (Tokyo) 1991;110:812–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

