Functional characterization and categorization of missense mutations that cause methylmalonyl-CoA mutase (MUT) deficiency
- PMID: 25125334
- PMCID: PMC4441004
- DOI: 10.1002/humu.22633
Functional characterization and categorization of missense mutations that cause methylmalonyl-CoA mutase (MUT) deficiency
Abstract
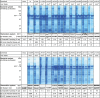
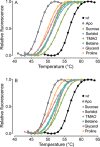
Methylmalonyl-CoA mutase (MUT) is an essential enzyme in propionate catabolism that requires adenosylcobalamin as a cofactor. Almost 250 inherited mutations in the MUT gene are known to cause the devastating disorder methylmalonic aciduria; however, the mechanism of dysfunction of these mutations, more than half of which are missense changes, has not been thoroughly investigated. Here, we examined 23 patient missense mutations covering a spectrum of exonic/structural regions, clinical phenotypes, and ethnic populations in order to determine their influence on protein stability, using two recombinant expression systems and a thermostability assay, and enzymatic function by measuring MUT activity and affinity for its cofactor and substrate. Our data stratify MUT missense mutations into categories of biochemical defects, including (1) reduced protein level due to misfolding, (2) increased thermolability, (3) impaired enzyme activity, and (4) reduced cofactor response in substrate turnover. We further demonstrate the stabilization of wild-type and thermolabile mutants by chemical chaperones in vitro and in bacterial cells. This in-depth mutation study illustrates the tools available for MUT enzyme characterization, guides future categorization of further missense mutations, and supports the development of alternative, chaperone-based therapy for patients not responding to current treatment.
Keywords: MUT; cobalamin; methylmalonic aciduria; methylmalonyl-CoA mutase; thermolability.
© 2014 WILEY PERIODICALS, INC.
Figures


References
-
- Acquaviva C, Benoist JF, Pereira S, Callebaut I, Koskas T, Porquet D, Elion J. 2005. Molecular basis of methylmalonyl‐CoA mutase apoenzyme defect in 40 European patients affected by mut(o) and mut‐forms of methylmalonic acidemia: identification of 29 novel mutations in the MUT gene. Hum Mutat 25:167–76. - PubMed
-
- Banerjee R, Ragsdale SW. 2003. The many faces of vitamin B12: catalysis by cobalamin‐dependent enzymes. Annu Rev Biochem 72:209–247. - PubMed
-
- Baumgartner R. 1983. Activity of the cobalamin‐dependent methylmalonyl‐<styled-content style="fixed-case">C</styled-content>o<styled-content style="fixed-case">A</styled-content> mutase In: Hall CA, editor. The cobalamins – volume 10 of methods in hematology. Churchill Livingstone, London, UK: p 181–193.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

