Tomato fruit chromoplasts behave as respiratory bioenergetic organelles during ripening
- PMID: 25125503
- PMCID: PMC4213118
- DOI: 10.1104/pp.114.243931
Tomato fruit chromoplasts behave as respiratory bioenergetic organelles during ripening
Abstract
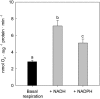
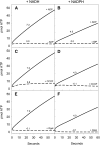
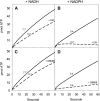
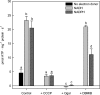
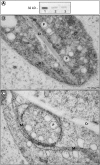
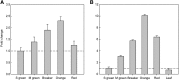
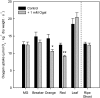
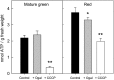
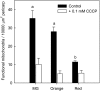
During tomato (Solanum lycopersicum) fruit ripening, chloroplasts differentiate into photosynthetically inactive chromoplasts. It was recently reported that tomato chromoplasts can synthesize ATP through a respiratory process called chromorespiration. Here we show that chromoplast oxygen consumption is stimulated by the electron donors NADH and NADPH and is sensitive to octyl gallate (Ogal), a plastidial terminal oxidase inhibitor. The ATP synthesis rate of isolated chromoplasts was dependent on the supply of NAD(P)H and was fully inhibited by Ogal. It was also inhibited by the proton uncoupler carbonylcyanide m-chlorophenylhydrazone, suggesting the involvement of a chemiosmotic gradient. In addition, ATP synthesis was sensitive to 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone, a cytochrome b6f complex inhibitor. The possible participation of this complex in chromorespiration was supported by the detection of one of its components (cytochrome f) in chromoplasts using immunoblot and immunocytochemical techniques. The observed increased expression of cytochrome c6 during ripening suggests that it could act as electron acceptor of the cytochrome b6f complex in chromorespiration. The effects of Ogal on respiration and ATP levels were also studied in tissue samples. Oxygen uptake of mature green fruit and leaf tissues was not affected by Ogal, but was inhibited increasingly in fruit pericarp throughout ripening (up to 26% in red fruit). Similarly, Ogal caused a significant decrease in ATP content of red fruit pericarp. The number of energized mitochondria, as determined by confocal microscopy, strongly decreased in fruit tissue during ripening. Therefore, the contribution of chromoplasts to total fruit respiration appears to increase in late ripening stages.
© 2014 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Backhausen JE, Vetter S, Baalmann E, Kitzmann C, Scheibe R. (1998) NAD-dependent malate dehydrogenase and glyceraldehyde 3-phosphate dehydrogenase isoenzymes play an important role in dark metabolism of various plastid types. Planta 205: 359–366
-
- Barr J, White WS, Chen L, Bae H, Rodermel S. (2004) The GHOST terminal oxidase regulates developmental programming in tomato fruit. Plant Cell Environ 27: 840–852
-
- Barsan C, Sanchez-Bel P, Rombaldi C, Egea I, Rossignol M, Kuntz M, Zouine M, Latché A, Bouzayen M, Pech JC. (2010) Characteristics of the tomato chromoplast revealed by proteomic analysis. J Exp Bot 61: 2413–2431 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

