Transgenic mice expressing mutated Tyr437His human myocilin develop progressive loss of retinal ganglion cell electrical responsiveness and axonopathy with normal iop
- PMID: 25125600
- PMCID: PMC4160076
- DOI: 10.1167/iovs.14-14793
Transgenic mice expressing mutated Tyr437His human myocilin develop progressive loss of retinal ganglion cell electrical responsiveness and axonopathy with normal iop
Abstract
Purpose: To characterize age-related changes of retinal ganglion cell (RGC) function, IOP, and anatomical markers of axon/glia integrity in a transgenic mouse expressing Tyr437His mutant of human myocilin protein.
Methods: Retinal ganglion cell electrical responsiveness was tested with pattern electroretinogram (PERG) in 11 transgenic mice expressing mutated myocilin at different ages over 18 months under ketamine/xylazine anesthesia. Twelve age-matched C57BL/6J mice also were tested as controls. Intraocular pressure was measured with a Tonolab tonometer. Immunohistochemistry for GFAP and neurofilament was performed on dissected optic nerve heads.
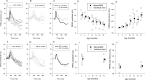
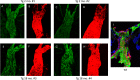
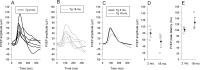
Results: In transgenic mice expressing mutated myocilin, the PERG amplitude progressively decreased with increasing age by approximately 50%, whereas the PERG peak latency increased by approximately 40 ms (ANOVA, P < 0.05). In contrast, PERGs of young and old control mice had similar amplitudes and peak latencies. In transgenic mice, GFAP staining was more intense and extended than in control mice, and increased with increasing age; neurofilament staining showed swollen and partially degenerated axons in old transgenic mice. The IOP of young transgenic mice was similar to that of control mice and did not significantly change with increasing age.
Conclusions: Transgenic mice expressing mutated human myocilin display progressive age-related changes in RGC electrical responsiveness that are not associated with IOP elevation but are associated with marked astrogliosis and axonopathy. Our results support the view that MYOC expression in the optic nerve may impact structural, metabolic, or neurotrophic support to RGC axons, thereby influencing their susceptibility to glaucomatous damage independently of IOP.
Keywords: intraocular pressure; mouse; myocylin; optic nerve; pattern electroretinogram; retinal ganglion cell.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

