Podocyte pathology and nephropathy - sphingolipids in glomerular diseases
- PMID: 25126087
- PMCID: PMC4115628
- DOI: 10.3389/fendo.2014.00127
Podocyte pathology and nephropathy - sphingolipids in glomerular diseases
Abstract
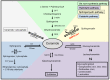
Sphingolipids are components of the lipid rafts in plasma membranes, which are important for proper function of podocytes, a key element of the glomerular filtration barrier. Research revealed an essential role of sphingolipids and sphingolipid metabolites in glomerular disorders of genetic and non-genetic origin. The discovery that glucocerebrosides accumulate in Gaucher disease in glomerular cells and are associated with clinical proteinuria initiated intensive research into the function of other sphingolipids in glomerular disorders. The accumulation of sphingolipids in other genetic diseases including Tay-Sachs, Sandhoff, Fabry, hereditary inclusion body myopathy 2, Niemann-Pick, and nephrotic syndrome of the Finnish type and its implications with respect to glomerular pathology will be discussed. Similarly, sphingolipid accumulation occurs in glomerular diseases of non-genetic origin including diabetic kidney disease (DKD), HIV-associated nephropathy, focal segmental glomerulosclerosis (FSGS), and lupus nephritis. Sphingomyelin metabolites, such as ceramide, sphingosine, and sphingosine-1-phosphate have also gained tremendous interest. We recently described that sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b) is expressed in podocytes where it modulates acid sphingomyelinase activity and acts as a master modulator of danger signaling. Decreased SMPDL3b expression in post-reperfusion kidney biopsies from transplant recipients with idiopathic FSGS correlates with the recurrence of proteinuria in patients and in experimental models of xenotransplantation. Increased SMPDL3b expression is associated with DKD. The consequences of differential SMPDL3b expression in podocytes in these diseases with respect to their pathogenesis will be discussed. Finally, the role of sphingolipids in the formation of lipid rafts in podocytes and their contribution to the maintenance of a functional slit diaphragm in the glomerulus will be discussed.
Keywords: ASMase; S1P; SMPDL3b; ceramide; glomerular disease; kidney disease; podocyte; sphingolipid.
Figures
References
-
- McIlwain H. The second thudichum lecture. Cerebral isolates and neurochemical discovery. Biochem Soc Trans (1975) 3:579–90 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

