Cytoplasmic nanojunctions between lysosomes and sarcoplasmic reticulum are required for specific calcium signaling
- PMID: 25126414
- PMCID: PMC4126599
- DOI: 10.12688/f1000research.3720.1
Cytoplasmic nanojunctions between lysosomes and sarcoplasmic reticulum are required for specific calcium signaling
Abstract
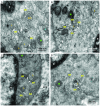
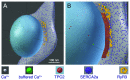
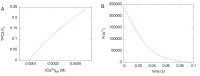
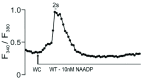
Herein we demonstrate how nanojunctions between lysosomes and sarcoplasmic reticulum (L-SR junctions) serve to couple lysosomal activation to regenerative, ryanodine receptor-mediated cellular Ca (2+) waves. In pulmonary artery smooth muscle cells (PASMCs) it has been proposed that nicotinic acid adenine dinucleotide phosphate (NAADP) triggers increases in cytoplasmic Ca (2+) via L-SR junctions, in a manner that requires initial Ca (2+) release from lysosomes and subsequent Ca (2+)-induced Ca (2+) release (CICR) via ryanodine receptor (RyR) subtype 3 on the SR membrane proximal to lysosomes. L-SR junction membrane separation has been estimated to be < 400 nm and thus beyond the resolution of light microscopy, which has restricted detailed investigations of the junctional coupling process. The present study utilizes standard and tomographic transmission electron microscopy to provide a thorough ultrastructural characterization of the L-SR junctions in PASMCs. We show that L-SR nanojunctions are prominent features within these cells and estimate that the junctional membrane separation and extension are about 15 nm and 300 nm, respectively. Furthermore, we develop a quantitative model of the L-SR junction using these measurements, prior kinetic and specific Ca (2+) signal information as input data. Simulations of NAADP-dependent junctional Ca (2+) transients demonstrate that the magnitude of these signals can breach the threshold for CICR via RyR3. By correlation analysis of live cell Ca (2+) signals and simulated Ca (2+) transients within L-SR junctions, we estimate that "trigger zones" comprising 60-100 junctions are required to confer a signal of similar magnitude. This is compatible with the 110 lysosomes/cell estimated from our ultrastructural observations. Most importantly, our model shows that increasing the L-SR junctional width above 50 nm lowers the magnitude of junctional [Ca (2+)] such that there is a failure to breach the threshold for CICR via RyR3. L-SR junctions are therefore a pre-requisite for efficient Ca (2+)signal coupling and may contribute to cellular function in health and disease.
Conflict of interest statement
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

