ERdj3 is an endoplasmic reticulum degradation factor for mutant glucocerebrosidase variants linked to Gaucher's disease
- PMID: 25126989
- PMCID: PMC4136546
- DOI: 10.1016/j.chembiol.2014.06.008
ERdj3 is an endoplasmic reticulum degradation factor for mutant glucocerebrosidase variants linked to Gaucher's disease
Abstract
Gaucher's disease (GD) is caused by mutations that compromise β-glucocerebrosidase (GCase) folding in the endoplasmic reticulum (ER), leading to excessive degradation instead of trafficking, which results in insufficient lysosomal function. We hypothesized that ER GCase interacting proteins play critical roles in making quality control decisions, i.e., facilitating ER-associated degradation (ERAD) instead of folding and trafficking. Utilizing GCase immunoprecipitation followed by mass-spectrometry-based proteomics, we identified endogenous HeLa cell GCase protein interactors, including ERdj3, an ER resident Hsp40 not previously established to interact with GCase. Depleting ERdj3 reduced the rate of mutant GCase degradation in patient-derived fibroblasts, while increasing folding, trafficking, and function by directing GCase to the profolding ER calnexin pathway. Inhibiting ERdj3-mediated mutant GCase degradation while simultaneously enhancing calnexin-associated folding, by way of a diltiazem-mediated increase in ER Ca(2+) levels, yields a synergistic rescue of L444P GCase lysosomal function. Our findings suggest a combination therapeutic strategy for ameliorating GD.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
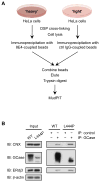
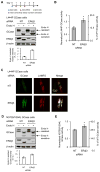

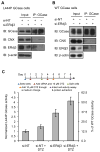
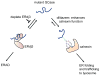
Comment in
-
Rehabilitating mutant GCase.Chem Biol. 2014 Aug 14;21(8):919-20. doi: 10.1016/j.chembiol.2014.07.010. Chem Biol. 2014. PMID: 25126987
Similar articles
-
Rehabilitating mutant GCase.Chem Biol. 2014 Aug 14;21(8):919-20. doi: 10.1016/j.chembiol.2014.07.010. Chem Biol. 2014. PMID: 25126987
-
Alteration of the proteostasis network of plant cells promotes the post-endoplasmic reticulum trafficking of recombinant mutant (L444P) human β-glucocerebrosidase.Plant Signal Behav. 2014;9(3):e28714. doi: 10.4161/psb.28714. Epub 2014 Apr 8. Plant Signal Behav. 2014. PMID: 24713615 Free PMC article.
-
Remodeling the proteostasis network to rescue glucocerebrosidase variants by inhibiting ER-associated degradation and enhancing ER folding.PLoS One. 2013 Apr 19;8(4):e61418. doi: 10.1371/journal.pone.0061418. Print 2013. PLoS One. 2013. PMID: 23620750 Free PMC article.
-
Gaucher disease paradigm: from ERAD to comorbidity.Hum Mutat. 2012 Oct;33(10):1398-407. doi: 10.1002/humu.22124. Epub 2012 Jun 11. Hum Mutat. 2012. PMID: 22623374 Review.
-
Enhancing the Activity of Glucocerebrosidase as a Treatment for Parkinson Disease.CNS Drugs. 2020 Sep;34(9):915-923. doi: 10.1007/s40263-020-00746-0. CNS Drugs. 2020. PMID: 32607746 Review.
Cited by
-
SDF2-like protein 1 (SDF2L1) regulates the endoplasmic reticulum localization and chaperone activity of ERdj3 protein.J Biol Chem. 2019 Dec 13;294(50):19335-19348. doi: 10.1074/jbc.RA119.009603. Epub 2019 Oct 17. J Biol Chem. 2019. PMID: 31624144 Free PMC article.
-
Control of Protein Homeostasis in the Early Secretory Pathway: Current Status and Challenges.Cells. 2019 Oct 29;8(11):1347. doi: 10.3390/cells8111347. Cells. 2019. PMID: 31671908 Free PMC article. Review.
-
Protein Quality Control in the Endoplasmic Reticulum.Protein J. 2019 Jun;38(3):317-329. doi: 10.1007/s10930-019-09831-w. Protein J. 2019. PMID: 31004255 Free PMC article. Review.
-
Modulation of calreticulin expression reveals a novel exosome-mediated mechanism of Z variant α1-antitrypsin disposal.J Biol Chem. 2019 Apr 19;294(16):6240-6252. doi: 10.1074/jbc.RA118.006142. Epub 2019 Mar 4. J Biol Chem. 2019. PMID: 30833329 Free PMC article.
-
Adapting Secretory Proteostasis and Function Through the Unfolded Protein Response.Curr Top Microbiol Immunol. 2018;414:1-25. doi: 10.1007/82_2017_56. Curr Top Microbiol Immunol. 2018. PMID: 28929194 Free PMC article. Review.
References
-
- Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct. 2008;33:75–89. - PubMed
-
- Aerts JM, Donker-Koopman WE, Murray GJ, Barranger JA, Tager JM, Schram AW. A procedure for the rapid purification in high yield of human glucocerebrosidase using immunoaffinity chromatography with monoclonal antibodies. Anal Biochem. 1986;154:655–663. - PubMed
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Barneveld RA, Tegelaers FP, Ginns EI, Visser P, Laanen EA, Brady RO, Galjaard H, Barranger JA, Reuser AJ, Tager JM. Monoclonal antibodies against human beta-glucocerebrosidase. Eur J Biochem. 1983;134:585–589. - PubMed
-
- Bendikov-Bar I, Horowitz M. Gaucher disease paradigm: from ERAD to comorbidity. Hum Mutat. 2012;33:1398–1407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

