Simplified follow-up after medical abortion using a low-sensitivity urinary pregnancy test and a pictorial instruction sheet in Rajasthan, India--study protocol and intervention adaptation of a randomised control trial
- PMID: 25127545
- PMCID: PMC4141880
- DOI: 10.1186/1472-6874-14-98
Simplified follow-up after medical abortion using a low-sensitivity urinary pregnancy test and a pictorial instruction sheet in Rajasthan, India--study protocol and intervention adaptation of a randomised control trial
Abstract
Background: The World Health Organisation suggests that simplification of the medical abortion regime will contribute to an increased acceptability of medical abortion, among women as well as providers. It is expected that a home-based follow-up after a medical abortion will increase the willingness to opt for medical abortion as well as decrease the workload and service costs in the clinic.
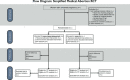
Methods/design: This study protocol describes a study that is a randomised, controlled, non-superiority trial. Women screened to participate in the study are those with unwanted pregnancies and gestational ages equal to or less than nine weeks. The randomisation list will be generated using a computerized random number generator and opaque sealed envelopes with group allocation will be prepared. Randomization of the study participants will occur after the first clinical encounter with the doctor. Eligible women randomised to the home-based assessment group will use a low-sensitivity pregnancy test and a pictorial instruction sheet at home, while the women in the clinic follow-up group will return to the clinic for routine follow-up carried out by a doctor. The primary objective of the study this study protocol describes is to evaluate the efficacy of home-based assessment using a low-sensitivity pregnancy test and a pictorial instruction sheet 10-14 days after an early medical abortion. Providers or research assistants will not be blinded during outcome assessment. To ensure feasibility of the self-assessment intervention an adaption phase took place at the selected study sites before study initiation. This resulted in an optimized, tailor-made intervention and in the development of the pictorial instruction sheet with a guide on how to use the low-sensitivity pregnancy test and the danger signs after a medical abortion.
Discussion: In this paper, we will describe the study protocol for a randomised control trial investigating the efficacy of simplified follow-up in terms of home-based assessment, 10-14 days after a medical abortion. Moreover, a description of the adaptation phase is included for a better understanding of the implementation of the intervention in a setting where literacy is low and the road-connections are poor.
Trial registration: Clinicaltrials.gov NCT01827995. Registered 04 May 2013.
Figures

Similar articles
-
Acceptability of Home-Assessment Post Medical Abortion and Medical Abortion in a Low-Resource Setting in Rajasthan, India. Secondary Outcome Analysis of a Non-Inferiority Randomized Controlled Trial.PLoS One. 2015 Sep 1;10(9):e0133354. doi: 10.1371/journal.pone.0133354. eCollection 2015. PLoS One. 2015. PMID: 26327217 Free PMC article. Clinical Trial.
-
Self-assessment of the outcome of early medical abortion versus clinic follow-up in India: a randomised, controlled, non-inferiority trial.Lancet Glob Health. 2015 Sep;3(9):e537-45. doi: 10.1016/S2214-109X(15)00150-3. Lancet Glob Health. 2015. PMID: 26275330 Clinical Trial.
-
Self-administered multi-level pregnancy tests in simplified follow-up of medical abortion in Tunisia.BMC Womens Health. 2016 Jul 30;16:49. doi: 10.1186/s12905-016-0327-1. BMC Womens Health. 2016. PMID: 27475998 Free PMC article. Clinical Trial.
-
Follow-up strategies to confirm the success of medical abortion of pregnancies up to 10 weeks' gestation: a systematic review with meta-analyses.Am J Obstet Gynecol. 2020 Jun;222(6):551-563.e13. doi: 10.1016/j.ajog.2019.11.1244. Epub 2019 Nov 9. Am J Obstet Gynecol. 2020. PMID: 31715147
-
Expulsion at home for early medical abortion: A systematic review with meta-analyses.Acta Obstet Gynecol Scand. 2021 Apr;100(4):727-735. doi: 10.1111/aogs.14025. Epub 2020 Nov 28. Acta Obstet Gynecol Scand. 2021. PMID: 33063314
Cited by
-
"Who Wants to Go Repeatedly to the Hospital?" Perceptions and Experiences of Simplified Medical Abortion in Rajasthan, India.Glob Qual Nurs Res. 2016 Dec 19;3:2333393616683073. doi: 10.1177/2333393616683073. eCollection 2016 Jan-Dec. Glob Qual Nurs Res. 2016. PMID: 28462355 Free PMC article.
-
Negotiating Collective and Individual Agency: A Qualitative Study of Young Women's Reproductive Health in Rural India.Qual Health Res. 2017 Feb;27(3):311-324. doi: 10.1177/1049732315613038. Epub 2016 Jul 11. Qual Health Res. 2017. PMID: 26531879 Free PMC article.
-
The importance of considering the evidence in the MTP 2014 Amendment debate in India - unsubstantiated arguments should not impede improved access to safe abortion.Glob Health Action. 2015 Mar 30;8:27512. doi: 10.3402/gha.v8.27512. eCollection 2015. Glob Health Action. 2015. PMID: 25828071 Free PMC article.
-
How task-sharing in abortion care became the norm in Sweden: A case study of historic and current determinants and events.Int J Gynaecol Obstet. 2020 Jul;150 Suppl 1(Suppl 1):34-42. doi: 10.1002/ijgo.13003. Int J Gynaecol Obstet. 2020. PMID: 33219992 Free PMC article.
-
Acceptability of Home-Assessment Post Medical Abortion and Medical Abortion in a Low-Resource Setting in Rajasthan, India. Secondary Outcome Analysis of a Non-Inferiority Randomized Controlled Trial.PLoS One. 2015 Sep 1;10(9):e0133354. doi: 10.1371/journal.pone.0133354. eCollection 2015. PLoS One. 2015. PMID: 26327217 Free PMC article. Clinical Trial.
References
-
- Sample Registration System (SRS) Maternal & Child Mortality and Total Fertility Rates. New Delhi: Office of Registrar General, India; 2011.
-
- Sample Registration System (SRS) Special Bulleting On Maternal Mortality In India 2010-12. New Delhi: Office of Registrar General, India; 2013. pp. 1–4.
-
- Sample Registration System (SRS) Maternal Mortality in India: 1997–2003 - Trends, Causes and Risk factors. New Delhi: Office of Registrar General, India; 2006. p. ii–29.
-
- Elul B, Bracken H, Verma S, Ved R, Singhi R, Lockwood K. Unwanted Pregnancy and Induced Abortion in Rajasthan, India: A Qualitative Exploration. New Delhi: Population Council, India; 2004. pp. 1–32.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

