Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-κB
- PMID: 25127718
- PMCID: PMC4246449
- DOI: 10.1186/1472-6882-14-299
Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-κB
Abstract
Background: Annona muricata leaves have been reported to have antiproliferative effects against various cancer cell lines. However, the detailed mechanism has yet to be defined. The current study was designed to evaluate the molecular mechanisms of A. muricata leaves ethyl acetate extract (AMEAE) against lung cancer A549 cells.
Methods: The effect of AMEAE on cell proliferation of different cell lines was analyzed by MTT assay. High content screening (HCS) was applied to investigate the suppression of NF-κB translocation, cell membrane permeability, mitochondrial membrane potential (MMP) and cytochrome c translocation from mitochondria to cytosol. Reactive oxygen species (ROS) formation, lactate dehydrogenase (LDH) release and activation of caspase-3/7, -8 and -9 were measured while treatment. The western blot analysis also carried out to determine the protein expression of cleaved caspase-3 and -9. Flow cytometry analysis was used to determine the cell cycle distribution and phosphatidylserine externalization. Quantitative PCR analysis was performed to measure the gene expression of Bax and Bcl-2 proteins.
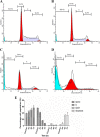
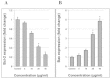
Results: Cell viability analysis revealed the selective cytotoxic effect of AMEAE towards lung cancer cells, A549, with an IC50 value of 5.09 ± 0.41 μg/mL after 72 h of treatment. Significant LDH leakage and phosphatidylserine externalization were observed in AMEAE treated cells by fluorescence analysis. Treatment of A549 cells with AMEAE significantly elevated ROS formation, followed by attenuation of MMP via upregulation of Bax and downregulation of Bcl-2, accompanied by cytochrome c release to the cytosol. The incubation of A549 cells with superoxide dismutase and catalase significantly attenuated the cytotoxicity caused by AMEAE, indicating that intracellular ROS plays a pivotal role in cell death. The released cytochrome c triggered the activation of caspase-9 followed by caspase-3. In addition, AMEAE-induced apoptosis was accompanied by cell cycle arrest at G0/G1 phase. Moreover, AMEAE suppressed the induced translocation of NF-κB from cytoplasm to nucleus.
Conclusions: Our data showed for the first time that the ethyl acetate extract of Annona muricata inhibited the proliferation of A549 cells, leading to cell cycle arrest and programmed cell death through activation of the mitochondrial-mediated signaling pathway with the involvement of the NF-kB signalling pathway.
Figures







Similar articles
-
Annona muricata leaves induce G₁ cell cycle arrest and apoptosis through mitochondria-mediated pathway in human HCT-116 and HT-29 colon cancer cells.J Ethnopharmacol. 2014 Oct 28;156:277-89. doi: 10.1016/j.jep.2014.08.011. Epub 2014 Sep 4. J Ethnopharmacol. 2014. PMID: 25195082
-
The chemopotential effect of Annona muricata leaves against azoxymethane-induced colonic aberrant crypt foci in rats and the apoptotic effect of Acetogenin Annomuricin E in HT-29 cells: a bioassay-guided approach.PLoS One. 2015 Apr 10;10(4):e0122288. doi: 10.1371/journal.pone.0122288. eCollection 2015. PLoS One. 2015. PMID: 25860620 Free PMC article.
-
Dentatin isolated from Clausena excavata induces apoptosis in MCF-7 cells through the intrinsic pathway with involvement of NF-κB signalling and G0/G1 cell cycle arrest: a bioassay-guided approach.J Ethnopharmacol. 2013 Jan 9;145(1):343-54. doi: 10.1016/j.jep.2012.11.020. Epub 2012 Nov 23. J Ethnopharmacol. 2013. PMID: 23178663
-
Chloroform Fraction of Methanolic Extract of Seeds of Annona muricata Induce S Phase Arrest and ROS Dependent Caspase Activated Mitochondria-Mediated Apoptosis in Triple-Negative Breast Cancer.Anticancer Agents Med Chem. 2021;21(10):1250-1265. doi: 10.2174/1871520620666200918101448. Anticancer Agents Med Chem. 2021. PMID: 32951586
-
Chamaejasmine induces apoptosis in human lung adenocarcinoma A549 cells through a Ros-mediated mitochondrial pathway.Molecules. 2011 Sep 27;16(10):8165-80. doi: 10.3390/molecules16108165. Molecules. 2011. PMID: 21952498 Free PMC article.
Cited by
-
Anticancer and Apoptogenic Effect of Graviola and Low-Dose Radiation in Tumor Xenograft in Mice.Integr Cancer Ther. 2020 Jan-Dec;19:1534735419900930. doi: 10.1177/1534735419900930. Integr Cancer Ther. 2020. PMID: 32493124 Free PMC article.
-
Acetogenins from Annona muricata as potential inhibitors of antiapoptotic proteins: a molecular modeling study.Drug Des Devel Ther. 2016 Apr 13;10:1399-410. doi: 10.2147/DDDT.S103216. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27110097 Free PMC article.
-
The Annona muricata leaf ethanol extract affects mobility and reproduction in mutant strain NB327 Caenorhabditis elegans.Biochem Biophys Rep. 2017 Apr 24;10:282-286. doi: 10.1016/j.bbrep.2017.04.016. eCollection 2017 Jul. Biochem Biophys Rep. 2017. PMID: 28955756 Free PMC article.
-
Quantitative Chemical Proteomics Reveals Resveratrol Inhibition of A549 Cell Migration Through Binding Multiple Targets to Regulate Cytoskeletal Remodeling and Suppress EMT.Front Pharmacol. 2021 Mar 26;12:636213. doi: 10.3389/fphar.2021.636213. eCollection 2021. Front Pharmacol. 2021. PMID: 33867987 Free PMC article.
-
LCMS/MS Phytochemical Profiling, Molecular, Pathological, and Immune-Histochemical Studies on the Anticancer Properties of Annona muricata.Molecules. 2023 Jul 29;28(15):5744. doi: 10.3390/molecules28155744. Molecules. 2023. PMID: 37570713 Free PMC article.
References
-
- Ramezanpour M, da Silva KB, Sanderson BJ. Venom present in sea anemone (heteractis magnifica) induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria-mediated pathway. Biotechnol Lett. 2012;36(3):1–7. - PubMed
-
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoopa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA-Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. - DOI - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1472-6882/14/299/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

