Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function
- PMID: 25128167
- PMCID: PMC4200341
- DOI: 10.1152/ajpheart.00328.2014
Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function
Abstract
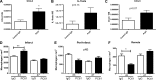
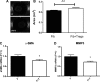
Regulatory T cells (Tregs) play a pivotal role in suppressing immune responses regulating behavior and gene expression in effector T cells, macrophages, and dendritic cells. Tregs infiltrate the infarcted myocardium; however, their role the inflammatory and reparative response after myocardial infarction remains poorly understood. We used FoxP3(EGFP) reporter mice to study Treg trafficking in the infarcted heart and examined the effects of Treg depletion on postinfarction remodeling using an anti-CD25 antibody. Moreover, we investigated the in vitro effects of Tregs on cardiac fibroblast phenotype and function. Low numbers of Tregs infiltrated the infarcted myocardium after 24-72 h of reperfusion. Treg depletion had no significant effects on cardiac dysfunction and scar size after reperfused myocardial infarction but accelerated ventricular dilation and accentuated apical remodeling. Enhanced myocardial dilation in Treg-depleted animals was associated with increased expression of chemokine (C-C motif) ligand 2 and accentuated macrophage infiltration. In vitro, Tregs modulated the cardiac fibroblast phenotype, reducing expression of α-smooth muscle actin, decreasing expression of matrix metalloproteinase-3, and attenuating contraction of fibroblast-populated collagen pads. Our findings suggest that endogenous Tregs have modest effects on the inflammatory and reparative response after myocardial infarction. However, the anti-inflammatory and matrix-preserving properties of Tregs may suggest a role for Treg-based cell therapy in the attenuation of adverse postinfarction remodeling.
Keywords: fibroblast; inflammation; lymphocyte; myocardial infarction; remodeling.
Copyright © 2014 the American Physiological Society.
Figures




References
-
- Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 116: 2127–2138, 2007 - PubMed
-
- Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-κB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation 103: 2296–2302, 2001 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

