Application and assessment of optical clearing methods for imaging of tissue-engineered neural stem cell spheres
- PMID: 25128373
- PMCID: PMC4346659
- DOI: 10.1089/ten.TEC.2014.0296
Application and assessment of optical clearing methods for imaging of tissue-engineered neural stem cell spheres
Abstract
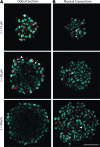
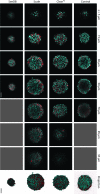
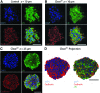
Three-dimensional (3D) cell culture is an important tool that facilitates biological discoveries by bridging the divide between standard two-dimensional cell culture and the complex, high-cell-density in vivo environment. Typically, the internal structures of 3D tissue-engineered samples are visualized through an involved process of physical sectioning, immunostaining, imaging, and computational reconstruction. However, recent progress in tissue-clearing methods has improved optical-imaging-depth capabilities in whole embryos and brains by reducing tissue opacity and light scattering, thus decreasing the need for physical sectioning. In this study, we assessed the application of the recently published clearing techniques Clear(T2), Scale, and SeeDB to tissue-engineered neural spheres. We found that scaffold-free self-assembled adult hippocampal neural stem cell spheres of 100-μm diameter could be optically cleared and imaged using either Clear(T2) or Scale, while SeeDB only marginally improved imaging depth. The Clear(T2) protocol maintained sphere size, while Scale led to sample expansion, and SeeDB led to sample shrinkage. Additionally, using Clear(T2) we cleared and successfully imaged spheres of C6 glioma cells and spheres of primary cortical neurons. We conclude that Clear(T2) is the most effective protocol of those tested at clearing neural spheres of various cell types and could be applied to better understand neural cell interactions in 3D tissue-engineered samples.
Figures


References
-
- Nakatomi H., Kuriu T., Okabe S., Yamamoto S., Hatano O., Kawahara N., Tamura A., Kirino T., and Nakafuku M.Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110,429, 2002 - PubMed
-
- Salman H., Ghosh P., and Kernie S.G.Subventricular zone neural stem cells remodel the brain following traumatic injury in adult mice. J Neurotrauma 21,283, 2004 - PubMed
-
- Doetsch F.A niche for adult neural stem cells. Curr Opin Genet Dev 13,543, 2003 - PubMed
-
- Temple S.The development of neural stem cells. Nature 414,112, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

