Activated microglia contribute to neuronal apoptosis in Toxoplasmic encephalitis
- PMID: 25128410
- PMCID: PMC4143554
- DOI: 10.1186/1756-3305-7-372
Activated microglia contribute to neuronal apoptosis in Toxoplasmic encephalitis
Abstract
Background: A plethora of evidence shows that activated microglia play a critical role in the pathogenesis of the central nervous system (CNS). Toxoplasmic encephalitis (TE) frequently occurs in HIV/AIDS patients. However, knowledge remains limited on the contributions of activated microglia to the pathogenesis of TE.
Methods: A murine model of reactivated encephalitis was generated in a latent infection with Toxoplasma gondii induced by cyclophosphamide. The neuronal apoptosis in the CNS and the profile of pro-inflammatory cytokines were assayed in both in vitro and in vivo experiments.
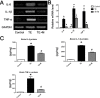
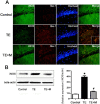
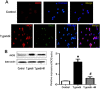
Results: Microglial cells were found to be activated in the cortex and hippocampus in the brain tissues of mice. The in vivo expression of interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and inducible nitric oxide synthase (iNOS) were up-regulated in TE mice, and accordingly, the neuronal apoptosis was significantly increased. The results were positively correlated with those of the in vitro experiments. Additionally,apoptosis of the mouse neuroblastoma type Neuro2a (N2a) remarkably increased when the N2a was co-cultured in transwell with microglial cells and Toxoplasma tachyzoites. Both in vivo and in vitro experiments showed that minocycline (a microglia inhibitor) treatment notably reduced microglial activation and neuronal apoptosis.
Conclusions: Activated microglia contribute to neuronal apoptosis in TE and inhibition of microglia activation might represent a novel therapeutic strategy of TE.
Figures





Similar articles
-
The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor.J Neuroinflammation. 2012 Sep 6;9:211. doi: 10.1186/1742-2094-9-211. J Neuroinflammation. 2012. PMID: 22950459 Free PMC article.
-
Regulation of microglia by CD4+ and CD8+ T cells: selective analysis in CD45-congenic normal and Toxoplasma gondii-infected bone marrow chimeras.Brain Pathol. 2001 Jan;11(1):44-55. doi: 10.1111/j.1750-3639.2001.tb00380.x. Brain Pathol. 2001. PMID: 11145203 Free PMC article.
-
Characteristics of Infection Immunity Regulated by Toxoplasma gondii to Maintain Chronic Infection in the Brain.Front Immunol. 2018 Feb 5;9:158. doi: 10.3389/fimmu.2018.00158. eCollection 2018. Front Immunol. 2018. PMID: 29459868 Free PMC article.
-
Galectin-3 and Galectin-9 May Differently Regulate the Expressions of Microglial M1/M2 Markers and T Helper 1/Th2 Cytokines in the Brains of Genetically Susceptible C57BL/6 and Resistant BALB/c Mice Following Peroral Infection With Toxoplasma gondii.Front Immunol. 2018 Jul 31;9:1648. doi: 10.3389/fimmu.2018.01648. eCollection 2018. Front Immunol. 2018. PMID: 30108583 Free PMC article.
-
Genes, cells and cytokines in resistance against development of toxoplasmic encephalitis.Immunobiology. 1999 Dec;201(2):255-71. doi: 10.1016/S0171-2985(99)80066-7. Immunobiology. 1999. PMID: 10631575 Review.
Cited by
-
Catastrophic consequences: can the feline parasite Toxoplasma gondii prompt the purrfect neuroinflammatory storm following traumatic brain injury?J Neuroinflammation. 2020 Jul 25;17(1):222. doi: 10.1186/s12974-020-01885-3. J Neuroinflammation. 2020. PMID: 32711529 Free PMC article. Review.
-
Lentinan has a beneficial effect on cognitive deficits induced by chronic Toxoplasma gondii infection in mice.Parasit Vectors. 2023 Dec 13;16(1):454. doi: 10.1186/s13071-023-06023-5. Parasit Vectors. 2023. PMID: 38093309 Free PMC article.
-
A hydrogel engineered to deliver minocycline locally to the injured cervical spinal cord protects respiratory neural circuitry and preserves diaphragm function.Neurobiol Dis. 2019 Jul;127:591-604. doi: 10.1016/j.nbd.2019.04.014. Epub 2019 Apr 25. Neurobiol Dis. 2019. PMID: 31028873 Free PMC article.
-
Encephalitis is mediated by ROP18 of Toxoplasma gondii, a severe pathogen in AIDS patients.Proc Natl Acad Sci U S A. 2018 Jun 5;115(23):E5344-E5352. doi: 10.1073/pnas.1801118115. Epub 2018 May 21. Proc Natl Acad Sci U S A. 2018. PMID: 29784816 Free PMC article.
-
HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment.Nat Rev Neurol. 2016 Oct 27;12(11):662-674. doi: 10.1038/nrneurol.2016.149. Nat Rev Neurol. 2016. PMID: 27786246 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

