Lack of angiopoietin-like-2 expression limits the metabolic stress induced by a high-fat diet and maintains endothelial function in mice
- PMID: 25128474
- PMCID: PMC4310393
- DOI: 10.1161/JAHA.114.001024
Lack of angiopoietin-like-2 expression limits the metabolic stress induced by a high-fat diet and maintains endothelial function in mice
Abstract
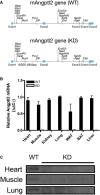
Background: Angiopoietin-like-2 (angptl2) is produced by several cell types including endothelial cells, adipocytes and macrophages, and contributes to the inflammatory process in cardiovascular diseases. We hypothesized that angptl2 impairs endothelial function, and that lowering angptl2 levels protects the endothelium against high-fat diet (HFD)-induced fat accumulation and hypercholesterolemia.
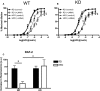
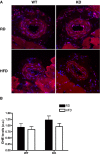
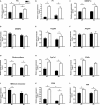
Methods and results: Acute recombinant angptl2 reduced (P<0.05) acetylcholine-mediated vasodilation of isolated wild-type (WT) mouse femoral artery, an effect reversed (P<0.05) by the antioxidant N-acetylcysteine. Accordingly, in angptl2 knockdown (KD) mice, ACh-mediated endothelium-dependent vasodilation was greater (P<0.05) than in WT mice. In arteries from KD mice, prostacyclin contributed to the overall dilation unlike in WT mice. After a 3-month HFD, overall vasodilation was not altered, but dissecting out the endothelial intrinsic pathways revealed that NO production was reduced in arteries isolated from HFD-fed WT mice (P<0.05), while NO release was maintained in KD mice. Similarly, endothelium-derived hyperpolarizing factor (EDHF) was preserved in mesenteric arteries from HFD-fed KD mice but not in those from WT mice. Finally, the HFD increased (P<0.05) total cholesterol-to-high-density lipoprotein ratios, low-density lipoprotein-to-high-density lipoprotein ratios, and leptin levels in WT mice only, while glycemia remained similar in the 2 strains. KD mice displayed less triglyceride accumulation in the liver (P<0.05 versus WT), and adipocyte diameters in mesenteric and epididymal white adipose tissues were smaller (P<0.05) in KD than in WT fed an HFD, while inflammatory gene expression increased (P<0.05) in the fat of WT mice only.
Conclusions: Lack of angptl2 expression limits the metabolic stress induced by an HFD and maintains endothelial function in mice.
Keywords: adipokines; endothelium‐derived relaxing factors; inflammation; isolated arteries.
© 2014 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures








References
-
- Reaven GM. Banting Lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988; 1988:1595-1607. - PubMed
-
- Krauss RM, Winston M, Fletcher RN, Grundy SM. Obesity: impact of cardiovascular disease. Circulation. 1998; 98:1472-1476. - PubMed
-
- Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y. Angiopoietin‐like protein 2 promotes chronic adipose tissue inflammation and obesity‐related systemic insulin resistance. Cell Metab. 2009; 10:178-188. - PubMed
-
- Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M, Tabata M, Tazume H, Tian Z, Takahashi O, Terada K, Takeya M, Hao H, Hirose N, Minami T, Suda T, Kiyohara Y, Ogawa H, Kaikita K, Oike Y. Role of endothelial cell‐derived Angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol. 2014; 34:790-800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

