Plasticity in the brainstem vagal circuits controlling gastric motor function triggered by corticotropin releasing factor
- PMID: 25128570
- PMCID: PMC4287746
- DOI: 10.1113/jphysiol.2014.278192
Plasticity in the brainstem vagal circuits controlling gastric motor function triggered by corticotropin releasing factor
Abstract
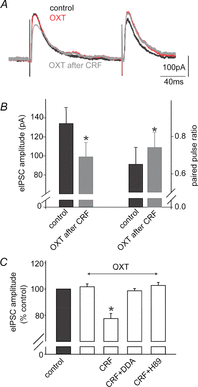
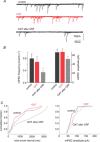
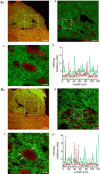
Stress impairs gastric emptying, reduces stomach compliance and induces early satiety via vagal actions. We have shown recently that the ability of the anti-stress neuropeptide oxytocin (OXT) to modulate vagal brainstem circuits undergoes short-term plasticity via alterations in cAMP levels subsequent to vagal afferent fibre-dependent activation of metabotropic glutamate receptors. The aim of the present study was to test the hypothesis that the OXT-induced gastric response undergoes plastic changes in the presence of the prototypical stress hormone, corticotropin releasing factor (CRF). Whole cell patch clamp recordings showed that CRF increased inhibitory GABAergic synaptic transmission to identified corpus-projecting dorsal motor nucleus of the vagus (DMV) neurones. In naive brainstem slices, OXT perfusion had no effect on inhibitory synaptic transmission; following exposure to CRF (and recovery from its actions), however, re-application of OXT inhibited GABAergic transmission in the majority of neurones tested. This uncovering of the OXT response was antagonized by pretreatment with protein kinase A or adenylate cyclase inhibitors, H89 and di-deoxyadenosine, respectively, indicating a cAMP-mediated mechanism. In naive animals, OXT microinjection in the dorsal vagal complex induced a NO-mediated corpus relaxation. Following CRF pretreatment, however, microinjection of OXT attenuated or, at times reversed, the gastric relaxation which was insensitive to l-NAME but was antagonized by pretreatment with a VIP antagonist. Immunohistochemical analyses of vagal motoneurones showed an increased number of oxytocin receptors present on GABAergic terminals of CRF-treated or stressed vs. naive rats. These results indicate that CRF alters vagal inhibitory circuits that uncover the ability of OXT to modulate GABAergic currents and modifies the gastric corpus motility response to OXT.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures

References
-
- Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Sustained acceleration of colonic transit following chronic homotypic stress in oxytocin knockout mice. Neurosci Lett. 2011;495:77–81. - PubMed
-
- Babygirija R, Bulbul M, Yoshimoto S, Ludwig K, Takahashi T. Central and peripheral release of oxytocin following chronic homotypic stress in rats. Auton Neurosci. 2012;167:56–60. - PubMed
-
- Babygirija R, Zheng J, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Sustained delayed gastric emptying during repeated restraint stress in oxytocin knockout mice. J Neuroendocrinol. 2010a;22:1181–1186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

